Expanding clinical laboratory tobacco product evaluation methods to loose-leaf tobacco vaporizers
- PMID: 27768968
- PMCID: PMC5140724
- DOI: 10.1016/j.drugalcdep.2016.10.005
Expanding clinical laboratory tobacco product evaluation methods to loose-leaf tobacco vaporizers
Abstract
Background: Novel tobacco products entering the US market include electronic cigarettes (ECIGs) and products advertised to "heat, not burn" tobacco. There is a growing literature regarding the acute effects of ECIGs. Less is known about "heat, not burn" products. This study's purpose was to expand existing clinical laboratory methods to examine, in cigarette smokers, the acute effects of a "heat, not burn" "loose-leaf tobacco vaporizer" (LLTV).
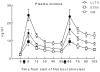
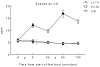
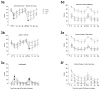
Methods: Plasma nicotine and breath carbon monoxide (CO) concentration and tobacco abstinence symptom severity were measured before and after two 10-puff (30-s interpuff interval) product use bouts separated by 60min. LLTV effects were compared to participants' own brand (OB) cigarettes and an ECIG (3.3V; 1.5ohm; 18mg/ml nicotine).
Results: Relative to OB, LLTV increased plasma nicotine concentration to a lesser degree, did not increase CO, and did not appear to reduce abstinence symptoms as effectively. Relative to ECIG, LLTV nicotine and CO delivery and abstinence symptom suppression did not differ. Participants reported that both the LLTV and ECIG were significantly less satisfying than OB.
Conclusions: Results demonstrate that LLTVs are capable of delivering nicotine and suppressing tobacco abstinence symptoms partially; acute effects of these products can be evaluated using existing clinical laboratory methods. Results can inform tobacco product regulation and may be predictive of the extent that these products have the potential to benefit or harm overall public health.
Keywords: Carbon monoxide; Electronic cigarette; Heat-not-burn products; Loose-leaf tobacco vaporizer; Plasma nicotine.
Copyright © 2016 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
No conflict declared.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

