Randomized controlled trials vs. observational studies: why not just live together?
- PMID: 27769172
- PMCID: PMC5073487
- DOI: 10.1186/s12871-016-0265-3
Randomized controlled trials vs. observational studies: why not just live together?
Abstract
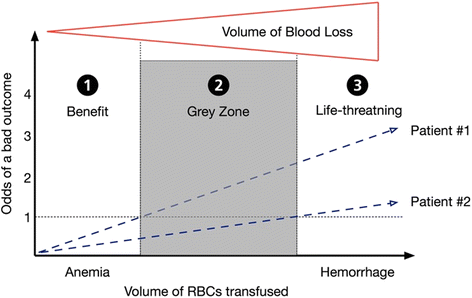
Randomized controlled trials (RCTs) are considered the gold standard for clinical research, thus having a high impact on clinical guidelines and our daily patients' care. However, various treatment strategies which we consider "evidence based" have never been subject to a prospective RCT, as we would rate it unethical to withheld an established treatment to individuals in an placebo controlled trial.In a recent BMC Anesthesiology publication, Trentino et al. analyzed the usefulness of observational studies in assessing benefit and risk of different transfusion strategies. The authors nicely reviewed and summarized similarities and differences, advantages and limitations, between different study types frequently used in transfusion medicine. In this interesting article, the authors conclude, that 'when comparing the results of observational studies with RCTs assessing transfusion outcomes, it is important that one consider not only the study method, but also the key elements of the study design'. Thus, in this commentary we now discuss the pro's and con's of different study types, even irrespective of transfusion medicine.
Keywords: Good clinical practice; Metaanalysis; Observational studies; Randomized controlled trials; Study planning.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous