Adding non-randomised studies to a Cochrane review brings complementary information for healthcare stakeholders: an augmented systematic review and meta-analysis
- PMID: 27769236
- PMCID: PMC5073845
- DOI: 10.1186/s12913-016-1816-5
Adding non-randomised studies to a Cochrane review brings complementary information for healthcare stakeholders: an augmented systematic review and meta-analysis
Abstract
Background: To reduce the burden of asthma, chronic disease management (CDM) programmes have been widely implemented and evaluated. Reviews including randomised controlled trials (RCTs) suggest that CDM programmes for asthma are effective. Other study designs are however often used for pragmatic reasons, but excluded from these reviews because of their design. We aimed to examine what complementary information could be retrieved from the addition of non-randomised studies to the studies included in a published Cochrane review on asthma CDM programmes, for healthcare stakeholders involved in the development, implementation, conduct or long-term sustainability of such programmes.
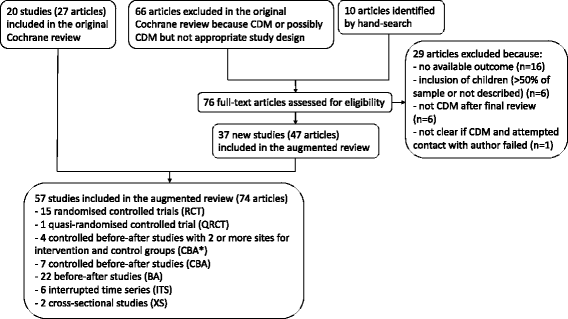
Methods: Extending a previously published Cochrane review, we performed a systematic review (augmented review) including any type of study designs instead of only those initially accepted by Cochrane and the Effective Practice and Organization of Care Review group. After double data selection and extraction, we compared study and intervention characteristics, assessed methodological quality and ran meta-analyses, by study design.
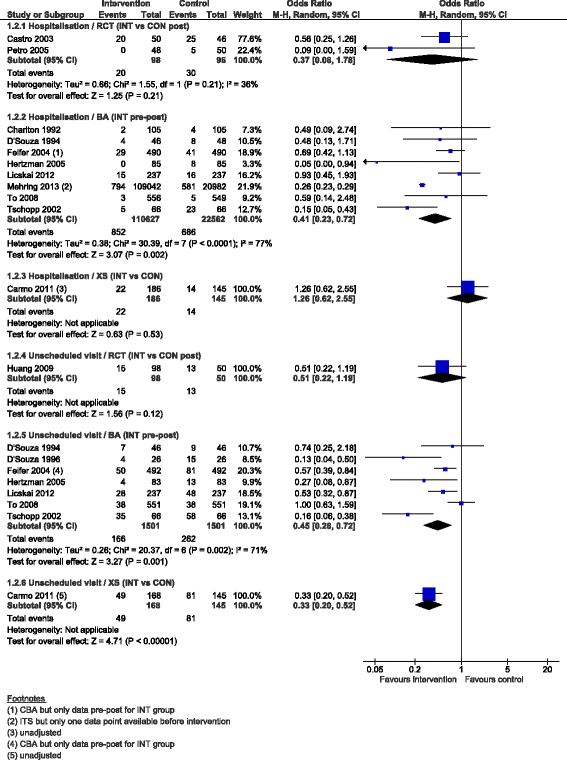
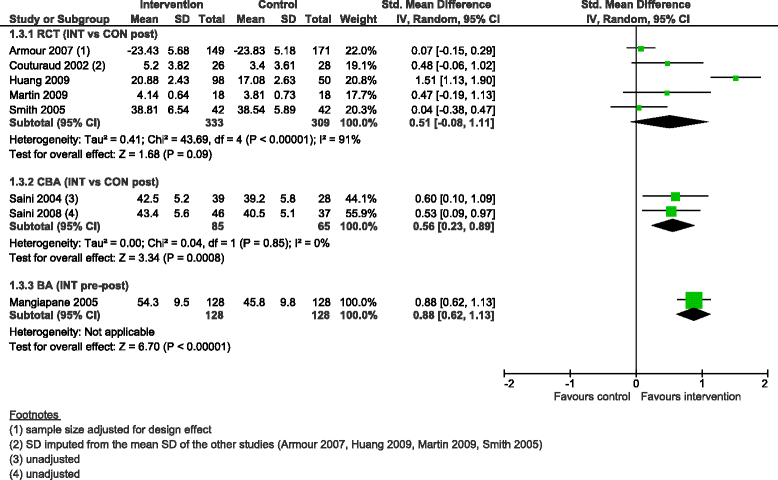
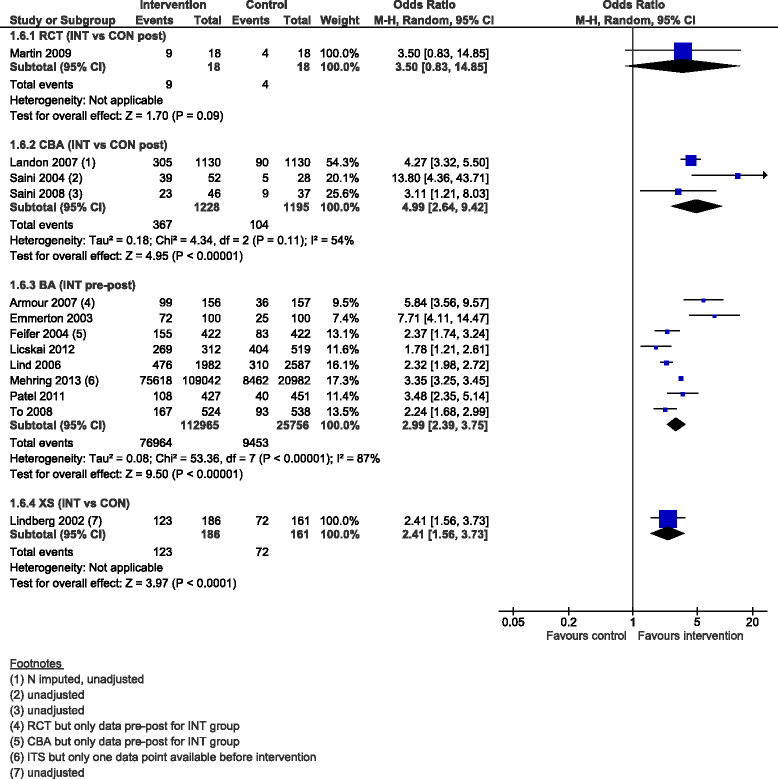
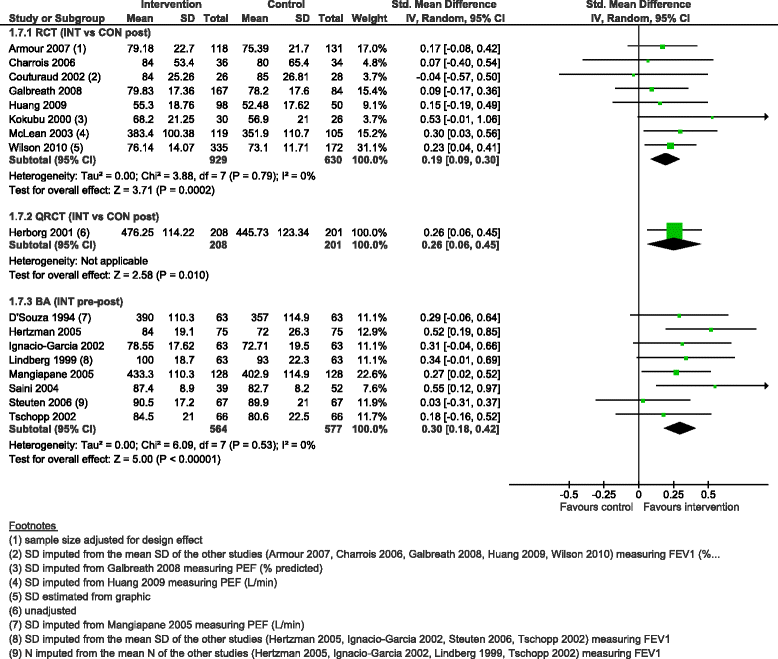
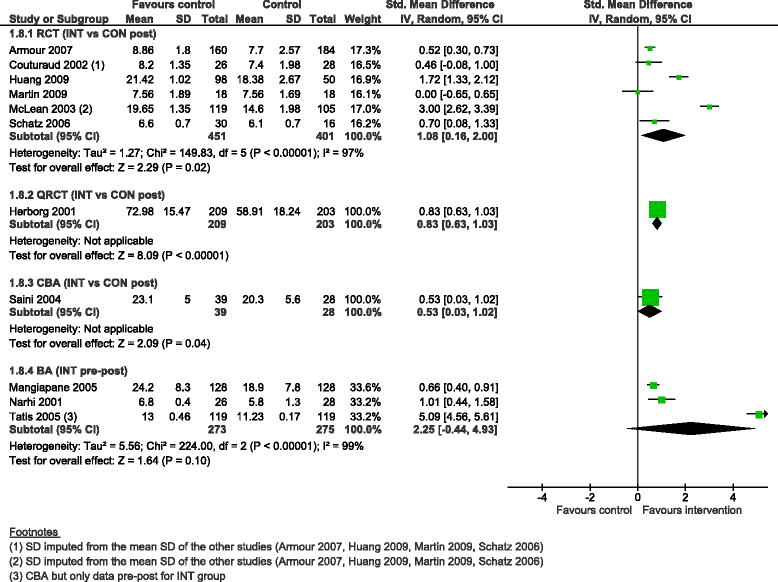
Results: We added 37 studies to the 20 studies included in the Cochrane review. The applicability of results was increased because of the larger variety of settings and asthma population considered. Also, adding non-randomised studies provided new evidence of improvements associated with CDM intervention (i.e. healthcare utilisation, days off work, use of action plan). Finally, evidence of CDM effectiveness in the added studies was consistent with the Cochrane review in terms of direction of effects.
Conclusions: The evidence of this augmented review is applicable to a broader set of patients and settings than those in the original Cochrane review. It also strengthens the message that CDM programmes have a beneficial effect on quality of life and disease severity, meaningful outcomes for the everyday life of patients with asthma. Despite the moderate to low methodological quality of all studies included, calling for caution in results interpretation and improvements in CDM evaluation methods and reporting, the inclusion of a broader set of study designs in systematic reviews of complex interventions, such as chronic disease management, is likely to be of high value and interest to patients, policymakers and other healthcare stakeholders.
Keywords: Asthma; Chronic disease management; Complex interventions; Meta-analysis; Study design; Systematic review.
Figures

 Low risk of bias;
Low risk of bias;  Unclear risk of bias;
Unclear risk of bias;  High risk of bias;
High risk of bias;  Not applicable; RCT: randomised controlled trial; QRCT: quasi-randomised controlled trial; CBA*: controlled before-after study (with at least two intervention and two control sites); CBA: controlled before-after study; ITS: interrupted-times-series study; BA: before-after study (without external control group); XS: cross-sectional study (with concurrent measurement of intervention exposure and outcomes)
Not applicable; RCT: randomised controlled trial; QRCT: quasi-randomised controlled trial; CBA*: controlled before-after study (with at least two intervention and two control sites); CBA: controlled before-after study; ITS: interrupted-times-series study; BA: before-after study (without external control group); XS: cross-sectional study (with concurrent measurement of intervention exposure and outcomes)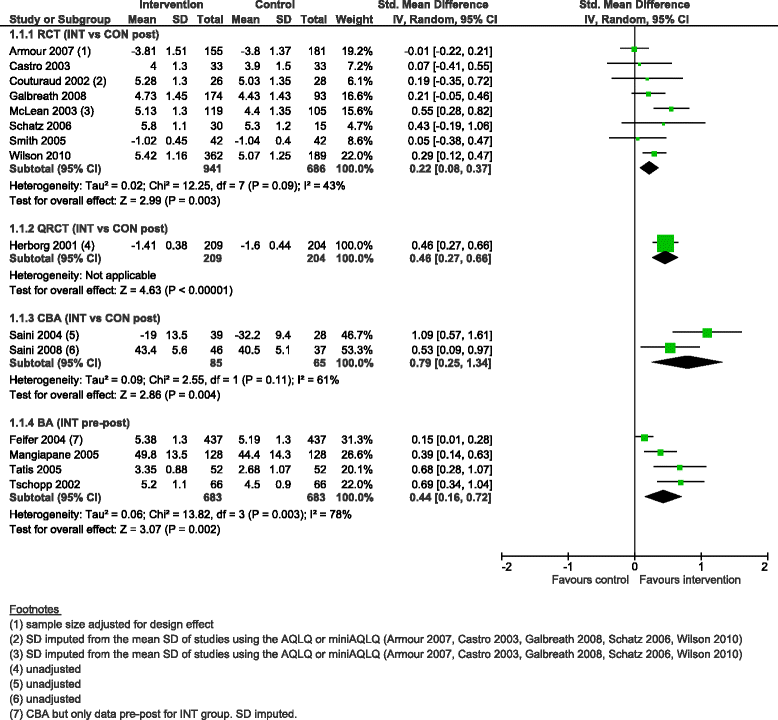



References
-
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. doi: 10.1016/S0140-6736(12)61728-0. - DOI - PMC - PubMed
-
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–96. doi: 10.1016/S0140-6736(12)61729-2. - DOI - PMC - PubMed
-
- Global Initative for Asthma. Global Strategy for Asthma Management and Prevention. 2015. www.ginasthma.org. Accessed 24 July 2015.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

