Clinical trials registries are underused in the pregnancy and childbirth literature: a systematic review of the top 20 journals
- PMID: 27769265
- PMCID: PMC5073738
- DOI: 10.1186/s13104-016-2280-3
Clinical trials registries are underused in the pregnancy and childbirth literature: a systematic review of the top 20 journals
Abstract
Background: Systematic reviews and meta-analyses that do not include unpublished data in their analyses may be prone to publication bias, which in some cases has been shown to have deleterious consequences on determining the efficacy of interventions.
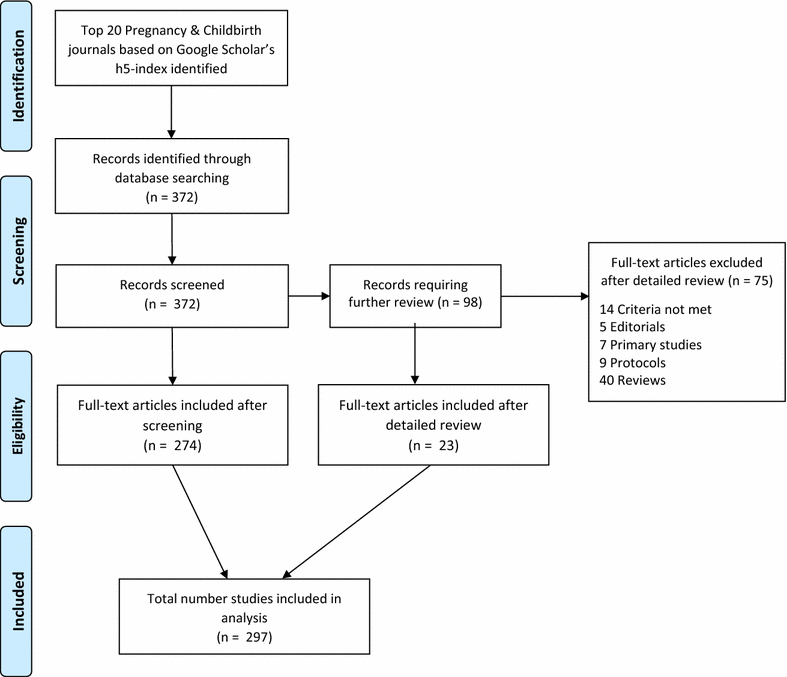
Methods: We retrieved systematic reviews and meta-analyses published in the past 8 years (January 1, 2007-December 31, 2015) from the top 20 journals in the Pregnancy and Childbirth literature, as rated by Google Scholar's h5-index. A meta-epidemiologic analysis was performed to determine the frequency with which authors searched clinical trials registries for unpublished data.
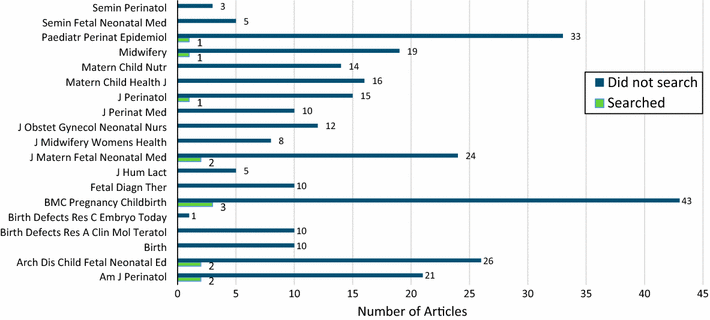
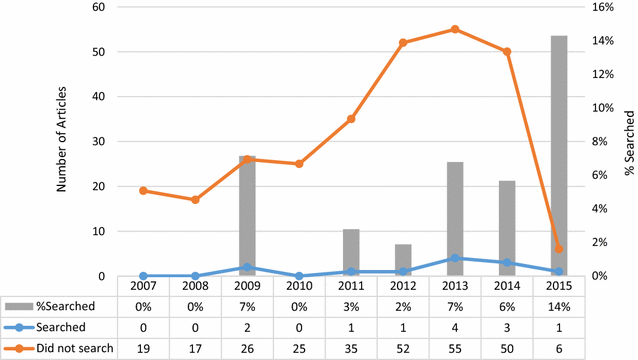
Results: A PubMed search retrieved 372 citations, 297 of which were deemed to be either a systematic review or a meta-analysis and were included for analysis. Twelve (4 %) of these searched at least one WHO-approved clinical trials registry or clinicaltrials.gov.
Conclusion: Systematic reviews and meta-analyses published in pregnancy and childbirth journals do not routinely report searches of clinical trials registries. Including these registries in systematic reviews may be a promising avenue to limit publication bias if registry searches locate unpublished trial data that could be used in the systematic review.
Keywords: Clinical trials registries; Obstetrics; Pregnancy and childbirth; Publication bias; Systematic review.
Figures
Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
-
Direct-acting antivirals for chronic hepatitis C.Cochrane Database Syst Rev. 2017 Sep 18;9(9):CD012143. doi: 10.1002/14651858.CD012143.pub3. Cochrane Database Syst Rev. 2017. PMID: 28922704 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Interventions for promoting habitual exercise in people living with and beyond cancer.Cochrane Database Syst Rev. 2018 Sep 19;9(9):CD010192. doi: 10.1002/14651858.CD010192.pub3. Cochrane Database Syst Rev. 2018. PMID: 30229557 Free PMC article.
Cited by
-
Inadequate diversity of information resources searched in US-affiliated systematic reviews and meta-analyses: 2005-2016.J Clin Epidemiol. 2018 Oct;102:50-62. doi: 10.1016/j.jclinepi.2018.05.024. Epub 2018 Jun 4. J Clin Epidemiol. 2018. PMID: 29879464 Free PMC article.
-
Research response to coronavirus disease 2019 needed better coordination and collaboration: a living mapping of registered trials.J Clin Epidemiol. 2021 Feb;130:107-116. doi: 10.1016/j.jclinepi.2020.10.010. Epub 2020 Oct 21. J Clin Epidemiol. 2021. PMID: 33096223 Free PMC article.
References
-
- Higgins J, Green S, Cochrane Collaboration . Cochrane handbook for systematic reviews of interventions Chichester. Hoboken: Wiley-Blackwell; 2011.
-
- National Institutes of Health: Trends, charts, and maps. (2015). https://www.clinicaltrials.gov/ct2/resources/trends. Accessed 31 Dec 2015.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

