Activation of HIV-1 expression in latently infected CD4+ T cells by the small molecule PKC412
- PMID: 27769267
- PMCID: PMC5073835
- DOI: 10.1186/s12985-016-0637-9
Activation of HIV-1 expression in latently infected CD4+ T cells by the small molecule PKC412
Abstract
Background: HIV-1 latency is a major obstacle for HIV-1 eradication. Extensive efforts are being directed toward the reactivation of latent HIV reservoirs with the aim of eliminating latently infected cells via the host immune system and/or virus-mediated cell lysis.
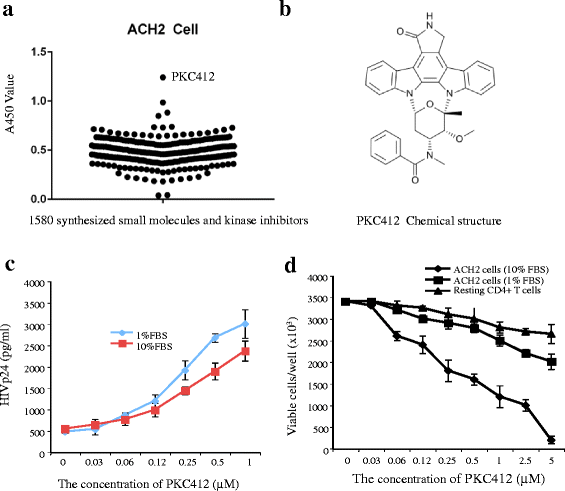
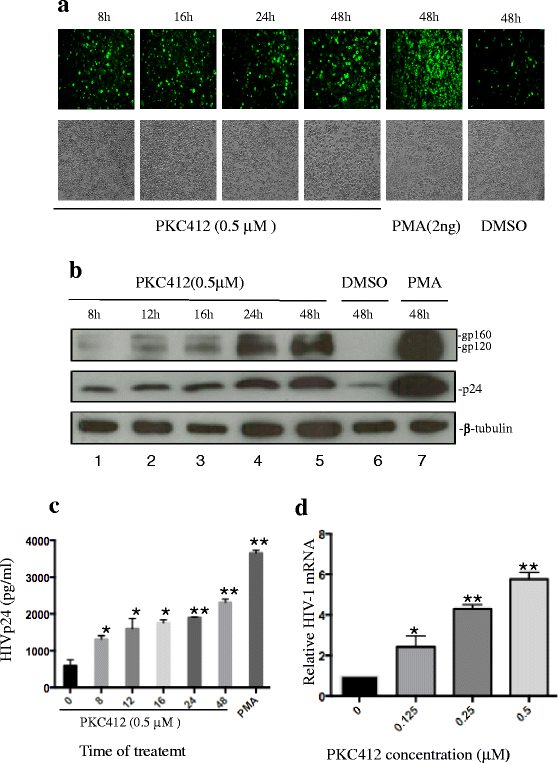
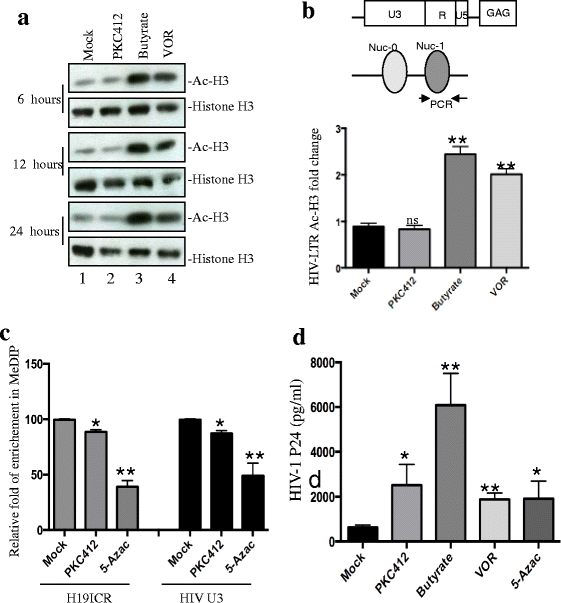
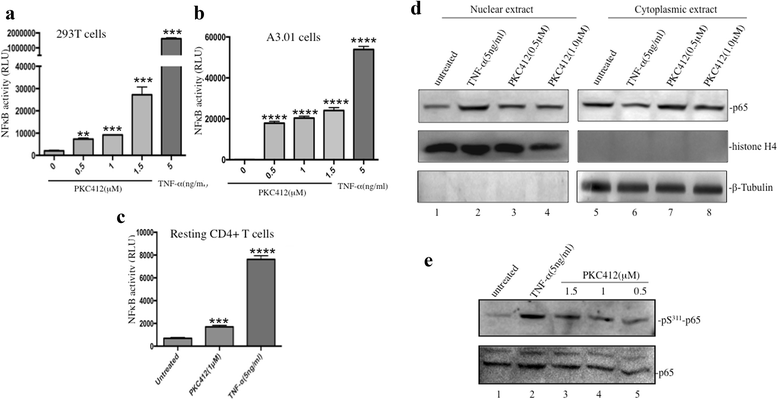
Results: We screened over 1,500 small molecules and kinase inhibitors and found that a small molecule, PKC412 (midostaurin, a broad-spectrum kinase inhibitor), can stimulate viral transcription and expression from the HIV-1 latently infected ACH2 cell line and primary resting CD4+ T cells. PKC412 reactivated HIV-1 expression in ACH2 cells in a dose- and time-dependent manner. Our results also suggest that the nuclear factor κB (NF-κB) signaling could be one of cellular pathways activated during PKC412-mediated activation of latent HIV-1 expression. Additionally, combining PKC412 with the HDAC inhibitor vorinostat (VOR) had an additive effect on HIV-1 reactivation in both ACH2 cells and infected resting CD4+ T cells.
Conclusions: These studies provide evidence that PKC412 is a new compound with the potential for optimization as a latency-reactivator to eradicate HIV-1 infection.
Keywords: ACH2 cells; HIV latency; NF-κB signaling; PKC412; Resting CD4+ T cells.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

