Not just an antibiotic target: Exploring the role of type I signal peptidase in bacterial virulence
- PMID: 27769673
- PMCID: PMC5279723
- DOI: 10.1016/j.bmc.2016.09.048
Not just an antibiotic target: Exploring the role of type I signal peptidase in bacterial virulence
Abstract
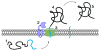
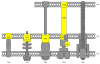
The looming antibiotic crisis has prompted the development of new strategies towards fighting infection. Traditional antibiotics target bacterial processes essential for viability, whereas proposed antivirulence approaches rely on the inhibition of factors that are required only for the initiation and propagation of infection within a host. Although antivirulence compounds have yet to prove their efficacy in the clinic, bacterial signal peptidase I (SPase) represents an attractive target in that SPase inhibitors exhibit broad-spectrum antibiotic activity, but even at sub-MIC doses also impair the secretion of essential virulence factors. The potential consequences of SPase inhibition on bacterial virulence have not been thoroughly examined, and are explored within this review. In addition, we review growing evidence that SPase has relevant biological functions outside of mediating secretion, and discuss how the inhibition of these functions may be clinically significant.
Keywords: Antivirulence; Arylomycin; Protein translocation; SPase; Secretion.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
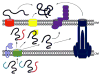

Similar articles
-
Inhibition of Protein Secretion in Escherichia coli and Sub-MIC Effects of Arylomycin Antibiotics.Antimicrob Agents Chemother. 2019 Jan 29;63(2):e01253-18. doi: 10.1128/AAC.01253-18. Print 2019 Feb. Antimicrob Agents Chemother. 2019. PMID: 30420476 Free PMC article.
-
The inhibition of type I bacterial signal peptidase: Biological consequences and therapeutic potential.Bioorg Med Chem Lett. 2015 Nov 1;25(21):4761-4766. doi: 10.1016/j.bmcl.2015.07.072. Epub 2015 Jul 26. Bioorg Med Chem Lett. 2015. PMID: 26276537 Free PMC article. Review.
-
Bacterial type I signal peptidase inhibitors - Optimized hits from nature.Eur J Med Chem. 2022 Aug 5;238:114490. doi: 10.1016/j.ejmech.2022.114490. Epub 2022 May 26. Eur J Med Chem. 2022. PMID: 35660251 Review.
-
Origins of Yersinia pestis sensitivity to the arylomycin antibiotics and the inhibition of type I signal peptidase.Antimicrob Agents Chemother. 2015 Jul;59(7):3887-98. doi: 10.1128/AAC.00181-15. Epub 2015 Apr 20. Antimicrob Agents Chemother. 2015. PMID: 25896690 Free PMC article.
-
Mechanism of action of the arylomycin antibiotics and effects of signal peptidase I inhibition.Antimicrob Agents Chemother. 2012 Oct;56(10):5054-60. doi: 10.1128/AAC.00785-12. Epub 2012 Jul 16. Antimicrob Agents Chemother. 2012. PMID: 22802255 Free PMC article.
Cited by
-
Involvement of signal peptidase I in Streptococcus sanguinis biofilm formation.Microbiology (Reading). 2017 Sep;163(9):1306-1318. doi: 10.1099/mic.0.000516. Epub 2017 Sep 4. Microbiology (Reading). 2017. PMID: 28869408 Free PMC article.
-
Repurposing human kinase inhibitors to create an antibiotic active against drug-resistant Staphylococcus aureus, persisters and biofilms.Nat Chem. 2020 Feb;12(2):145-158. doi: 10.1038/s41557-019-0378-7. Epub 2019 Dec 16. Nat Chem. 2020. PMID: 31844194 Free PMC article.
-
Inhibition of Protein Secretion in Escherichia coli and Sub-MIC Effects of Arylomycin Antibiotics.Antimicrob Agents Chemother. 2019 Jan 29;63(2):e01253-18. doi: 10.1128/AAC.01253-18. Print 2019 Feb. Antimicrob Agents Chemother. 2019. PMID: 30420476 Free PMC article.
-
A novel secretion and online-cleavage strategy for production of cecropin A in Escherichia coli.Sci Rep. 2017 Aug 4;7(1):7368. doi: 10.1038/s41598-017-07411-5. Sci Rep. 2017. PMID: 28779147 Free PMC article.
-
Regulatory mechanisms of sub-inhibitory levels antibiotics agent in bacterial virulence.Appl Microbiol Biotechnol. 2021 May;105(9):3495-3505. doi: 10.1007/s00253-021-11291-1. Epub 2021 Apr 24. Appl Microbiol Biotechnol. 2021. PMID: 33893838 Review.
References
-
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance. Geneva, Switzerland: WHO Document Production Services; 2014.
-
- Kostyanev T, Bonten MJ, O'Brien S, Steel H, Ross S, Francois B, Tacconelli E, Winterhalter M, Stavenger RA, Karlén A, Harbarth S, Hackett J, Jafri HS, Vuong C, MacGowan A, Witschi A, Angyalosi G, Elborn JS, deWinter R, Goossens H. J Antimicrob Chemother. 2016;71:290. - PubMed
-
- O'Neill J. Antimicrobial Resistance: Tackling a crisis for the Health and Wealth of Nations. London, UK: Review on Antimicrobial Resistance; 2014.
-
- Antibiotics Currently in Clinical Development. Philadelphia, PA: The Pew Charitable Trusts; 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

