Enzyme-mediated stiffening hydrogels for probing activation of pancreatic stellate cells
- PMID: 27769941
- PMCID: PMC5235985
- DOI: 10.1016/j.actbio.2016.10.027
Enzyme-mediated stiffening hydrogels for probing activation of pancreatic stellate cells
Abstract
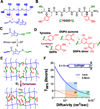
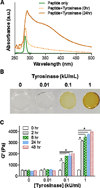
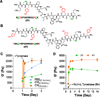
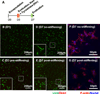
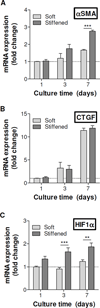
The complex network of biochemical and biophysical cues in the pancreatic desmoplasia not only presents challenges to the fundamental understanding of tumor progression, but also hinders the development of therapeutic strategies against pancreatic cancer. Residing in the desmoplasia, pancreatic stellate cells (PSCs) are the major stromal cells affecting the growth and metastasis of pancreatic cancer cells by means of paracrine effects and extracellular matrix protein deposition. PSCs remain in a quiescent/dormant state until they are 'activated' by various environmental cues. While the mechanisms of PSC activation are increasingly being described in literature, the influence of matrix stiffness on PSC activation is largely unexplored. To test the hypothesis that matrix stiffness affects myofibroblastic activation of PSCs, we have prepared cell-laden hydrogels capable of being dynamically stiffened through an enzymatic reaction. The stiffening of the microenvironment was created by using a peptide linker with additional tyrosine residues, which were susceptible to tyrosinase-mediated crosslinking. Tyrosinase catalyzes the oxidation of tyrosine into dihydroxyphenylalanine (DOPA), DOPA quinone, and finally into DOPA dimer. The formation of DOPA dimer led to additional crosslinks and thus stiffening the cell-laden hydrogel. In addition to systematically studying the various parameters relevant to the enzymatic reaction and hydrogel stiffening, we also designed experiments to probe the influence of dynamic matrix stiffening on cell fate. Protease-sensitive peptides were used to crosslink hydrogels, whereas integrin-binding ligands (e.g., RGD motif) were immobilized in the network to afford cell-matrix interaction. PSC-laden hydrogels were placed in media containing tyrosinase for 6h to achieve in situ gel stiffening. We found that PSCs encapsulated and cultured in a stiffened matrix expressed higher levels of αSMA and hypoxia-inducible factor 1α (HIF-1α), suggestive of a myofibroblastic phenotype. This hydrogel platform offers a facile means of in situ stiffening of cell-laden matrices and should be valuable for probing cell fate process dictated by dynamic matrix stiffness.
Statement of significance: Hydrogels with spatial-temporal controls over crosslinking kinetics (i.e., dynamic hydrogel) are increasingly being developed for studying mechanobiology in 3D. The general principle of designing dynamic hydrogel is to perform cell encapsulation within a hydrogel network that allows for postgelation modification in gel crosslinking density. The enzyme-mediated in situ gel stiffening is innovative because of the specificity and efficiency of enzymatic reaction. Although tyrosinase has been used for hydrogel crosslinking and in situ cell encapsulation, to the best of our knowledge tyrosinase-mediated DOPA formation has not been explored for in situ stiffening of cell-laden hydrogels. Furthermore, the current work provides a gradual matrix stiffening strategy that may more closely mimic the process of tumor development.
Keywords: Hydrogels; Pancreatic cancer; Stellate cells; Tissue stiffening; Tyrosinase.
Copyright © 2016 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures



References
-
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. - PubMed
-
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

