Duration of antibody response following vaccination against feline immunodeficiency virus
- PMID: 27770018
- PMCID: PMC11110993
- DOI: 10.1177/1098612X16673292
Duration of antibody response following vaccination against feline immunodeficiency virus
Abstract
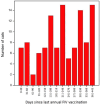
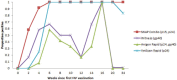
Objectives Recently, two point-of-care (PoC) feline immunodeficiency virus (FIV) antibody test kits (Witness and Anigen Rapid) were reported as being able to differentiate FIV-vaccinated from FIV-infected cats at a single time point, irrespective of the gap between testing and last vaccination (0-7 years). The aim of the current study was to investigate systematically anti-FIV antibody production over time in response to the recommended primary FIV vaccination series. Methods First, residual plasma from the original study was tested using a laboratory-based ELISA to determine whether negative results with PoC testing were due to reduced as opposed to absent antibodies to gp40. Second, a prospective study was performed using immunologically naive client-owned kittens and cats given a primary FIV vaccination series using a commercially available inactivated whole cell/inactivated whole virus vaccine (Fel-O-Vax FIV, three subcutaneous injections at 4 week intervals) and tested systematically (up to 11 times) over 6 months, using four commercially available PoC FIV antibody kits (SNAP FIV/FeLV Combo [detects antibodies to p15/p24], Witness FeLV/FIV [gp40], Anigen Rapid FIV/FeLV [p24/gp40] and VetScan FeLV/FIV Rapid [p24]). Results The laboratory-based ELISA showed cats from the original study vaccinated within the previous 0-15 months had detectable levels of antibodies to gp40, despite testing negative with two kits that use gp40 as a capture antigen (Witness and Anigen Rapid kits). The prospective study showed that antibody testing with SNAP Combo and VetScan Rapid was positive in all cats 2 weeks after the second primary FIV vaccination, and remained positive for the duration of the study (12/12 and 10/12 cats positive, respectively). Antibody testing with Witness and Anigen Rapid was also positive in a high proportion of cats 2 weeks after the second primary FIV vaccination (8/12 and 7/12, respectively), but antibody levels declined below the level of detection in most cats (10/12) by 1 month after the third (final) primary FIV vaccination. All cats tested negative using Witness and Anigen Rapid 6 months after the third primary FIV vaccination. Conclusions and relevance This study has shown that a primary course of FIV vaccination does not interfere with FIV antibody testing in cats using Witness and Anigen Rapid, provided primary vaccination has not occurred within the previous 6 months. Consequently, Witness and Anigen Rapid antibody test kits can be used reliably to determine FIV infection status at the time of annual booster FIV vaccination to help detect 'vaccine breakthroughs' and in cats that have not received a primary course of FIV vaccination within the preceding 6 months. The duration of antibody response following annual booster FIV vaccination and the resulting effect on antibody testing using PoC kits needs to be determined by further research. The mechanism(s) for the variation in FIV antibody test kit performance remains unclear.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Pedersen N, Ho E, Brown M, et al.. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science 1987; 235: 790–793. - PubMed
-
- Shelton GH, Grant CK, Cotter SM, et al.. Feline immunodeficiency virus and feline leukemia virus infections and their relationships to lymphoid malignancies in cats: a retrospective study (1968–1988). J Acquir Immune Defic Syndr 1990; 3: 623–630. - PubMed
-
- Lecollinet S, Richardson J. Vaccination against the feline immunodeficiency virus: the road not taken. Comp Immun Microbiol Infect Dis 2008; 31: 167–190. - PubMed
-
- Yamamoto JK, Pu RY, Sato E. Feline immunodeficiency virus pathogenesis and development of a dual-subtype feline-immunodeficiency-virus vaccine. AIDS 2007; 21: 547–563. - PubMed
-
- Bienzle D. FIV in cats – a useful model of HIV in people? Vet Immunol Immunopathol 2014; 159: 171–179. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

