Lysyl Oxidase-like-2 Cross-links Collagen IV of Glomerular Basement Membrane
- PMID: 27770022
- PMCID: PMC5207071
- DOI: 10.1074/jbc.M116.738856
Lysyl Oxidase-like-2 Cross-links Collagen IV of Glomerular Basement Membrane
Abstract
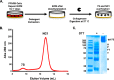
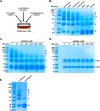
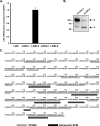
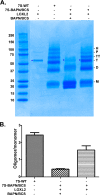
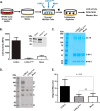
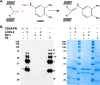
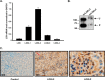
The 7S dodecamer is recognized as an important structural cross-linking domain of collagen IV networks that provide mechanical stability to basement membranes, a specialized form of extracellular matrix essential for the development and maintenance of tissue architecture. Although the 7S dodecamer is stabilized by covalent cross-linking, the molecular mechanism by which such cross-links are formed has not been revealed. Here, we aimed to identify the enzyme(s) that cross-links the 7S dodecamer and characterize its expression in the kidney glomerulus. Pharmacological inhibition of candidate extracellular matrix enzymes revealed that lysyl oxidase activity is required for cross-linking of 7S polypeptides. Among all lysyl oxidase family members, lysyl oxidase-like-2 (LOXL2) was identified as the isoform cross-linking collagen IV in mouse embryonal PFHR-9 cells. Biochemical analyses revealed that LOXL2 readily promoted the formation of lysyl-derived cross-links in the 7S dodecamer but not in the NC1 domain. We also established that LOXL2 is the main lysyl oxidase family member present in the glomerular extracellular matrix. Altogether, we demonstrate that LOXL2 is a novel component of the molecular machinery that forms cross-linked collagen IV networks, which are essential for glomerular basement membrane stability and molecular ultrafiltration function.
Keywords: basement membrane; collagen; extracellular matrix; kidney; lysyl oxidase; protein cross-linking.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
References
-
- Hudson B. G., Reeders S. T., and Tryggvason K. (1993) Type IV collagen: structure, gene organization, and role in human diseases. Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. J. Biol. Chem. 268, 26033–26036 - PubMed
-
- Boutaud A., Borza D.-B., Bondar O., Gunwar S., Netzer K.-O., Singh N., Ninomiya Y., Sado Y., Noelken M. E., and Hudson B. G. (2000) Type IV collagen of the glomerular basement membrane. Evidence that the chain specificity of network assembly is encoded by the noncollagenous NC1 domains. J. Biol. Chem. 275, 30716–30724 - PubMed
-
- Timpl R., Wiedemann H., van Delden V., Furthmayr H., and Kühn K. (1981) A network model for the organization of type IV collagen molecules in basement membranes. Eur. J. Biochem. 120, 203–211 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

