Cell-Type-Specific H+-ATPase Activity in Root Tissues Enables K+ Retention and Mediates Acclimation of Barley (Hordeum vulgare) to Salinity Stress
- PMID: 27770060
- PMCID: PMC5129721
- DOI: 10.1104/pp.16.01347
Cell-Type-Specific H+-ATPase Activity in Root Tissues Enables K+ Retention and Mediates Acclimation of Barley (Hordeum vulgare) to Salinity Stress
Abstract
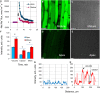
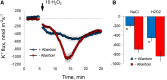
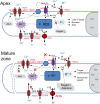
While the importance of cell type specificity in plant adaptive responses is widely accepted, only a limited number of studies have addressed this issue at the functional level. We have combined electrophysiological, imaging, and biochemical techniques to reveal the physiological mechanisms conferring higher sensitivity of apical root cells to salinity in barley (Hordeum vulgare). We show that salinity application to the root apex arrests root growth in a highly tissue- and treatment-specific manner. Although salinity-induced transient net Na+ uptake was about 4-fold higher in the root apex compared with the mature zone, mature root cells accumulated more cytosolic and vacuolar Na+, suggesting that the higher sensitivity of apical cells to salt is not related to either enhanced Na+ exclusion or sequestration inside the root. Rather, the above differential sensitivity between the two zones originates from a 10-fold difference in K+ efflux between the mature zone and the apical region (much poorer in the root apex) of the root. Major factors contributing to this poor K+ retention ability are (1) an intrinsically lower H+-ATPase activity in the root apex, (2) greater salt-induced membrane depolarization, and (3) a higher reactive oxygen species production under NaCl and a larger density of reactive oxygen species-activated cation currents in the apex. Salinity treatment increased (2- to 5-fold) the content of 10 (out of 25 detected) amino acids in the root apex but not in the mature zone and changed the organic acid and sugar contents. The causal link between the observed changes in the root metabolic profile and the regulation of transporter activity is discussed.
© 2016 American Society of Plant Biologists. All Rights Reserved.
Figures





References
-
- Anschütz U, Becker D, Shabala S (2014) Going beyond nutrition: regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J Plant Physiol 171: 670–687 - PubMed
-
- Chen Z, Newman I, Zhou M, Mendham N, Zhang G, Shabala S (2005) Screening plants for salt tolerance by measuring K+ flux: a case study for barley. Plant Cell Environ 28: 1230–1246
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

