Phase I/II trials of 186Re-HEDP in metastatic castration-resistant prostate cancer: post-hoc analysis of the impact of administered activity and dosimetry on survival
- PMID: 27770145
- PMCID: PMC5323472
- DOI: 10.1007/s00259-016-3543-x
Phase I/II trials of 186Re-HEDP in metastatic castration-resistant prostate cancer: post-hoc analysis of the impact of administered activity and dosimetry on survival
Abstract
Purpose: To investigate the role of patient-specific dosimetry as a predictive marker of survival and as a potential tool for individualised molecular radiotherapy treatment planning of bone metastases from castration-resistant prostate cancer, and to assess whether higher administered levels of activity are associated with a survival benefit.
Methods: Clinical data from 57 patients who received 2.5-5.1 GBq of 186Re-HEDP as part of NIH-funded phase I/II clinical trials were analysed. Whole-body and SPECT-based absorbed doses to the whole body and bone lesions were calculated for 22 patients receiving 5 GBq. The patient mean absorbed dose was defined as the mean of all bone lesion-absorbed doses in any given patient. Kaplan-Meier curves, log-rank tests, Cox's proportional hazards model and Pearson's correlation coefficients were used for overall survival (OS) and correlation analyses.
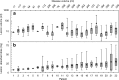
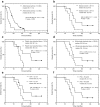
Results: A statistically significantly longer OS was associated with administered activities above 3.5 GBq in the 57 patients (20.1 vs 7.1 months, hazard ratio: 0.39, 95 % CI: 0.10-0.58, P = 0.002). A total of 379 bone lesions were identified in 22 patients. The mean of the patient mean absorbed dose was 19 (±6) Gy and the mean of the whole-body absorbed dose was 0.33 (±0.11) Gy for the 22 patients. The patient mean absorbed dose (r = 0.65, P = 0.001) and the whole-body absorbed dose (r = 0.63, P = 0.002) showed a positive correlation with disease volume. Significant differences in OS were observed for the univariate group analyses according to disease volume as measured from SPECT imaging of 186Re-HEDP (P = 0.03) and patient mean absorbed dose (P = 0.01), whilst only the disease volume remained significant in a multivariable analysis (P = 0.004).
Conclusion: This study demonstrated that higher administered activities led to prolonged survival and that for a fixed administered activity, the whole-body and patient mean absorbed doses correlated with the extent of disease, which, in turn, correlated with survival. This study shows the importance of patient stratification to establish absorbed dose-response correlations and indicates the potential to individualise treatment of bone metastases with radiopharmaceuticals according to patient-specific imaging and dosimetry.
Keywords: Bone metastases; Dosimetry; Molecular radiotherapy; Prostate cancer; Radiopharmaceutical; Survival.
Conflict of interest statement
Conflict of interest
The authors have no potential conflicts of interest relevant to the subject of this article
Ethical approval
This article does not contain any studies with animals performed by any of the authors. All patients provided written informed consent to take part in the trials, which were approved by the Royal Marsden NHS Foundation Trust and The Institute of Cancer Research Ethics committee.
Figures


Comment in
-
Single high dose versus repeated bone-targeted radionuclide therapy.Eur J Nucl Med Mol Imaging. 2017 Nov;44(12):2144-2145. doi: 10.1007/s00259-017-3815-0. Epub 2017 Aug 31. Eur J Nucl Med Mol Imaging. 2017. PMID: 28861597 No abstract available.
-
Reply to 'Single high dose versus repeated bone-targeted radionuclide therapy'.Eur J Nucl Med Mol Imaging. 2018 Mar;45(3):515-517. doi: 10.1007/s00259-017-3902-2. Eur J Nucl Med Mol Imaging. 2018. PMID: 29247283 No abstract available.
Similar articles
-
Bone lesion absorbed dose profiles in patients with metastatic prostate cancer treated with molecular radiotherapy.Br J Radiol. 2018 Apr;91(1084):20170795. doi: 10.1259/bjr.20170795. Epub 2018 Feb 5. Br J Radiol. 2018. PMID: 29293372 Free PMC article.
-
A model-based method for the prediction of whole-body absorbed dose and bone marrow toxicity for 186Re-HEDP treatment of skeletal metastases from prostate cancer.Eur J Nucl Med Mol Imaging. 2003 Aug;30(8):1114-24. doi: 10.1007/s00259-003-1197-y. Epub 2003 May 22. Eur J Nucl Med Mol Imaging. 2003. PMID: 12761596 Clinical Trial.
-
Dosimetry for (177)Lu-DKFZ-PSMA-617: a new radiopharmaceutical for the treatment of metastatic prostate cancer.Eur J Nucl Med Mol Imaging. 2016 Jan;43(1):42-51. doi: 10.1007/s00259-015-3174-7. Epub 2015 Aug 29. Eur J Nucl Med Mol Imaging. 2016. PMID: 26318602
-
Dosimetry of [177Lu]Lu-PSMA-Targeted Radiopharmaceutical Therapies in Patients with Prostate Cancer: A Comparative Systematic Review and Metaanalysis.J Nucl Med. 2024 Aug 1;65(8):1264-1271. doi: 10.2967/jnumed.124.267452. J Nucl Med. 2024. PMID: 38960712 Free PMC article.
-
Optimal usage of radium-223 in metastatic castration-resistant prostate cancer.J Formos Med Assoc. 2017 Nov;116(11):825-836. doi: 10.1016/j.jfma.2017.04.005. Epub 2017 Oct 16. J Formos Med Assoc. 2017. PMID: 29046247 Review.
Cited by
-
Bifunctional chelators for radiorhenium: past, present and future outlook.RSC Med Chem. 2022 Jan 14;13(3):217-245. doi: 10.1039/d1md00364j. eCollection 2022 Mar 23. RSC Med Chem. 2022. PMID: 35434629 Free PMC article. Review.
-
Theranostic radiopharmaceuticals: established agents in current use.Br J Radiol. 2018 Nov;91(1091):20170969. doi: 10.1259/bjr.20170969. Epub 2018 Mar 12. Br J Radiol. 2018. PMID: 29474096 Free PMC article. Review.
-
Single high dose versus repeated bone-targeted radionuclide therapy.Eur J Nucl Med Mol Imaging. 2017 Nov;44(12):2144-2145. doi: 10.1007/s00259-017-3815-0. Epub 2017 Aug 31. Eur J Nucl Med Mol Imaging. 2017. PMID: 28861597 No abstract available.
-
A radiobiological model of metastatic burden reduction for molecular radiotherapy: application to patients with bone metastases.Phys Med Biol. 2017 Apr 7;62(7):2859-2870. doi: 10.1088/1361-6560/aa5e6f. Phys Med Biol. 2017. PMID: 28291739 Free PMC article.
-
Imaging and dosimetry for radium-223: the potential for personalized treatment.Br J Radiol. 2017 Aug;90(1077):20160748. doi: 10.1259/bjr.20160748. Epub 2017 Jun 27. Br J Radiol. 2017. PMID: 28654303 Free PMC article. Review.
References
-
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBse No. 11 [Internet]. Internation Agency for Research on Cancer, Lyon, France. 2013. http://globocan.iarc.fr. Accessed December 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

