Chemosensitivity and Endocrine Sensitivity in Clinical Luminal Breast Cancer Patients in the Prospective Neoadjuvant Breast Registry Symphony Trial (NBRST) Predicted by Molecular Subtyping
- PMID: 27770345
- PMCID: PMC5306085
- DOI: 10.1245/s10434-016-5600-x
Chemosensitivity and Endocrine Sensitivity in Clinical Luminal Breast Cancer Patients in the Prospective Neoadjuvant Breast Registry Symphony Trial (NBRST) Predicted by Molecular Subtyping
Abstract
Purpose: Hormone receptor-positive (HR+) tumors have heterogeneous biology and present a challenge for determining optimal treatment. In the Neoadjuvant Breast Registry Symphony Trial (NBRST) patients were classified according to MammaPrint/BluePrint subtyping to provide insight into the response to neoadjuvant endocrine therapy (NET) or neoadjuvant chemotherapy (NCT).
Objective: The purpose of this predefined substudy was to compare MammaPrint/BluePrint with conventional 'clinical' immunohistochemistry/fluorescence in situ hybridization (IHC/FISH) subtyping in 'clinical luminal' [HR+/human epidermal growth factor receptor 2-negative (HER2-)] breast cancer patients to predict treatment sensitivity.
Methods: NBRST IHC/FISH HR+/HER2- breast cancer patients (n = 474) were classified into four molecular subgroups by MammaPrint/BluePrint subtyping: Luminal A, Luminal B, HER2, and Basal type. Pathological complete response (pCR) rates were compared with conventional IHC/FISH subtype.
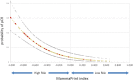
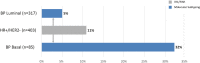
Results: The overall pCR rate for 'clinical luminal' patients to NCT was 11 %; however, 87 of these 474 patients were reclassified as Basal type by BluePrint, with a high pCR rate of 32 %. The MammaPrint index was highly associated with the likelihood of pCR (p < 0.001). Fifty-three patients with BluePrint Luminal tumors received NET with an aromatase inhibitor and 36 (68 %) had a clinical response.
Conclusions: With BluePrint subtyping, 18 % of clinical 'luminal' patients are classified in a different subgroup, compared with conventional assessment, and these patients have a significantly higher response rate to NCT compared with BluePrint Luminal patients. MammaPrint/BluePrint subtyping can help allocate effective treatment to appropriate patients. In addition, accurate identification of subtype biology is important in the interpretation of neoadjuvant treatment response since lack of pCR in luminal patients does not portend the worse prognosis associated with residual disease in Basal and HER2 subtypes.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

