Expression of scavenger receptor-AI promotes alternative activation of murine macrophages to limit hepatic inflammation and fibrosis
- PMID: 27770558
- PMCID: PMC5191952
- DOI: 10.1002/hep.28873
Expression of scavenger receptor-AI promotes alternative activation of murine macrophages to limit hepatic inflammation and fibrosis
Abstract
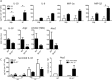
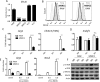
The liver maintains an immunologically tolerant environment as a result of continuous exposure to food and bacterial constituents from the digestive tract. Hepatotropic pathogens can take advantage of this niche and establish lifelong chronic infections causing hepatic fibrosis and hepatocellular carcinoma. Macrophages (Mϕ) play a critical role in regulation of immune responses to hepatic infection and regeneration of tissue. However, the factors crucial for Mϕ in limiting hepatic inflammation or resolving liver damage have not been fully understood. In this report, we demonstrate that expression of C-type lectin receptor scavenger receptor-AI (SR-AI) is crucial for promoting M2-like Mϕ activation and polarization during hepatic inflammation. Liver Mϕ uniquely up-regulated SR-AI during hepatotropic viral infection and displayed increased expression of alternative Mϕ activation markers, such as YM-1, arginase-1, and interleukin-10 by activation of mer receptor tyrosine kinase associated with inhibition of mammalian target of rapamycin. Expression of these molecules was reduced on Mϕ obtained from livers of infected mice deficient for the gene encoding SR-AI (msr1). Furthermore, in vitro studies using an SR-AI-deficient Mϕ cell line revealed impeded M2 polarization and decreased phagocytic capacity. Direct stimulation with virus was sufficient to activate M2 gene expression in the wild-type (WT) cell line, but not in the knockdown cell line. Importantly, tissue damage and fibrosis were exacerbated in SR-AI-/- mice following hepatic infection and adoptive transfer of WT bone-marrow-derived Mϕ conferred protection against fibrosis in these mice.
Conclusion: SR-AI expression on liver Mϕ promotes recovery from infection-induced tissue damage by mediating a switch to a proresolving Mϕ polarization state. (Hepatology 2017;65:32-43).
© 2016 The Authors. Hepatology published by Wiley Periodicals, Inc., on behalf of the American Association for the Study of Liver Diseases.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

