Effects of a novel β-lapachone derivative on Trypanosoma cruzi: Parasite death involving apoptosis, autophagy and necrosis
- PMID: 27770751
- PMCID: PMC5078628
- DOI: 10.1016/j.ijpddr.2016.10.003
Effects of a novel β-lapachone derivative on Trypanosoma cruzi: Parasite death involving apoptosis, autophagy and necrosis
Abstract
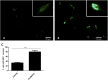
Natural products comprise valuable sources for new antiparasitic drugs. Here we tested the effects of a novel β-lapachone derivative on Trypanosoma cruzi parasite survival and proliferation and used microscopy and cytometry techniques to approach the mechanism(s) underlying parasite death. The selectivity index determination indicate that the compound trypanocidal activity was over ten-fold more cytotoxic to epimastigotes than to macrophages or splenocytes. Scanning electron microscopy analysis revealed that the R72 β-lapachone derivative affected the T. cruzi morphology and surface topography. General plasma membrane waving and blebbing particularly on the cytostome region were observed in the R72-treated parasites. Transmission electron microscopy observations confirmed the surface damage at the cytostome opening vicinity. We also observed ultrastructural evidence of the autophagic mechanism termed macroautophagy. Some of the autophagosomes involved large portions of the parasite cytoplasm and their fusion/confluence may lead to necrotic parasite death. The remarkably enhanced frequency of autophagy triggering was confirmed by quantitating monodansylcadaverine labeling. Some cells displayed evidence of chromatin pycnosis and nuclear fragmentation were detected. This latter phenomenon was also indicated by DAPI staining of R72-treated cells. The apoptotis induction was suggested to take place in circa one-third of the parasites assessed by annexin V labeling measured by flow cytometry. TUNEL staining corroborated the apoptosis induction. Propidium iodide labeling indicate that at least 10% of the R72-treated parasites suffered necrosis within 24 h. The present data indicate that the β-lapachone derivative R72 selectively triggers T. cruzi cell death, involving both apoptosis and autophagy-induced necrosis.
Keywords: Chagas disease; Chemotherapy; Natural products; Trypanosoma cruzi; β–lapachone derivative.
Copyright © 2016 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


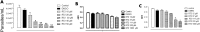







References
-
- Aguiar C., Batista A.M., Pavan T.B., Almeida E.A., Guariento M.E., Wanderley J.S., Costa Serological profiles and evaluation of parasitaemia by PCR and blood culture in individuals chronically infected by Trypanosoma cruzi treated with benzonidazole. Trop. Med. Int. Health. 2012;17:368–373. - PubMed
-
- Almeida M.T., Ramalho-Santos J., Oliveira C.R., de Lima M.C. Parameters affecting fusion between liposomes and synaptosomes. Role of proteins, lipid peroxidation, pH and temperature. J. Membr. Biol. 1994;142:217–222. - PubMed
-
- Alvarez V.E., Kosec G., Sant'Anna C., Turk V., Cazzulo J.J., Turk B. Autophagy is involved in nutritional stress response and differentiation in Trypanosoma cruzi. J. Biol. Chem. 2008;283:3454–3464. - PubMed
-
- Araújo A.J., De Souza A.A., Da Silva Júnior E.N., Marinho-Filho J.D., de Moura M.A., Rocha D.D., Vasconcellos M.C., Costa C.O., Pessoa C., Moraes De, Ferreira V.S., De Abreu F.C., Pinto A.V., Montenegro R.C., Costa-Lotufo L.V., Goulart M.O. Growth inhibitory effects of 3'-nitro-3-phenylamino nor-beta-lapachone against HL-60, a redox-dependent mechanism. Toxicol. In Vitro. 2012;26:585–589. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

