A randomised controlled trial of the clinical and cost-effectiveness of a contingency management intervention compared to treatment as usual for reduction of cannabis use and of relapse in early psychosis (CIRCLE): a study protocol for a randomised controlled trial
- PMID: 27770820
- PMCID: PMC5075422
- DOI: 10.1186/s13063-016-1620-x
A randomised controlled trial of the clinical and cost-effectiveness of a contingency management intervention compared to treatment as usual for reduction of cannabis use and of relapse in early psychosis (CIRCLE): a study protocol for a randomised controlled trial
Abstract
Background: Around 35-45 % of people in contact with services for a first episode of psychosis are using cannabis. Cannabis use is associated with delays in remission, poorer clinical outcomes, significant increases in the risk of relapse, and lower engagement in work or education. While there is a clear need for effective interventions, so far only very limited benefits have been achieved from psychological interventions. Contingency management (CM) is a behavioural intervention in which specified desired behavioural change is reinforced through financial rewards. CM is now recognised to have a substantial evidence base in some contexts and its adoption in the UK is advocated by the National Institute for Health and Care Excellence (NICE) guidance as a treatment for substance or alcohol misuse. However, there is currently little published data testing its effectiveness for reducing cannabis use in early psychosis.
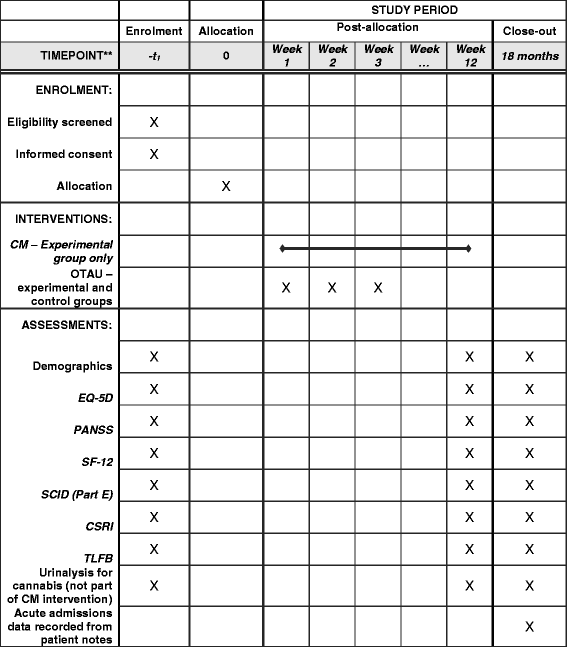
Methods: CIRCLE is a two-arm, rater-blinded randomised controlled trial (RCT) investigating the clinical and cost-effectiveness of a CM intervention for reducing cannabis use among young people receiving treatment from UK Early Intervention in Psychosis (EIP) services. EIP service users (n = 544) with a recent history of cannabis use will be recruited. The experimental group will receive 12 once-weekly CM sessions, and a voucher reward if urinalysis shows that they have not used cannabis in the previous week. Both the experimental and the control groups will be offered an Optimised Treatment as Usual (OTAU) psychoeducational package targeting cannabis use. Assessment interviews will be performed at consent, at 3 months, and at 18 months. The primary outcome is time to relapse, defined as admission to an acute mental health service. Secondary outcomes include proportion of cannabis-free urine samples during the intervention period, severity of positive psychotic symptoms, quality-adjusted life years, and engagement in work or education.
Discussion: CIRCLE is a RCT of CM for cannabis use in young people with a recent history of psychosis (EIP service users) and recent cannabis use. It is designed to investigate whether the intervention is a clinically and cost-effective treatment for cannabis use. It is intended to inform future treatment delivery, particularly in EIP settings.
Trial registration: ISRCTN33576045 : doi 10.1186/ISRCTN33576045 , registered on 28 November 2011.
Keywords: Cannabis; Contingency management; Early intervention; Financial incentives; Psychosis; Substance misuse.
Figures
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources