Integrating immunometabolism and macrophage diversity
- PMID: 27771140
- PMCID: PMC5333784
- DOI: 10.1016/j.smim.2016.10.004
Integrating immunometabolism and macrophage diversity
Abstract
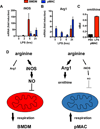
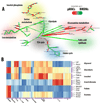
Macrophages are heterogeneous cells that play a key role in inflammatory and tissue reparative responses. Over the past decade it has become clear that shifts in cellular metabolism are important determinants of macrophage function and phenotype. At the same time, our appreciation of macrophage diversity in vivo has also been increasing. Factors such as cell origin and tissue localization are now recognized as important variables that influence macrophage biology. Whether different macrophage populations also have unique metabolic phenotypes has not been extensively explored. In this article, we will discuss the importance of understanding how macrophage origin can modulate metabolic programming and influence inflammatory responses.
Keywords: Glycolysis; Inflammation; Metabolism; Mitochondria; Nitric oxide.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures


References
-
- Jha AK, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42:419–430. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

