In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease
- PMID: 27771327
- PMCID: PMC5512932
- DOI: 10.1016/j.jtos.2016.09.004
In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease
Abstract
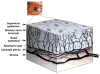
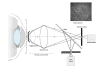
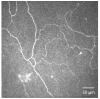
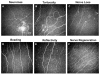
In vivo confocal microscopy (IVCM) is becoming an indispensable tool for studying corneal physiology and disease. Enabling the dissection of corneal architecture at a cellular level, this technique offers fast and noninvasive in vivo imaging of the cornea with images comparable to those of ex vivo histochemical techniques. Corneal nerves bear substantial relevance to clinicians and scientists alike, given their pivotal roles in regulation of corneal sensation, maintenance of epithelial integrity, as well as proliferation and promotion of wound healing. Thus, IVCM offers a unique method to study corneal nerve alterations in a myriad of conditions, such as ocular and systemic diseases and following corneal surgery, without altering the tissue microenvironment. Of particular interest has been the correlation of corneal subbasal nerves to their function, which has been studied in normal eyes, contact lens wearers, and patients with keratoconus, infectious keratitis, corneal dystrophies, and neurotrophic keratopathy. Longitudinal studies have applied IVCM to investigate the effects of corneal surgery on nerves, demonstrating their regenerative capacity. IVCM is increasingly important in the diagnosis and management of systemic conditions such as peripheral diabetic neuropathy and, more recently, in ocular diseases. In this review, we outline the principles and applications of IVCM in the study of corneal nerves in various ocular and systemic diseases.
Keywords: corneal dystrophies; corneal nerves; corneal sensitivity; corneal surgery; diabetes; in vivo confocal microscopy; keratoconus; pain; peripheral neuropathy.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no commercial or proprietary interest in any concept or product discussed in this article.
Figures









References
-
- Al-Aqaba MA, Fares U, Suleman H, et al. Architecture and distribution of human corneal nerves. Br J Ophthalmol. 2009;94:784–9. - PubMed
-
- Al-Aqaba MA, Alomar T, Miri A, et al. Ex vivo confocal microscopy of human corneal nerves. Br J Ophthalmol. 2010;94:1251–7. - PubMed
-
- Muller LJ, Vrensen GF, Pels L, et al. Architecture of human corneal nerves. Invest Ophthalmol Vis Sci. 1997;38:985–94. - PubMed
-
- Marfurt CF, Cox J, Deek S, Dvorscak L. Anatomy of the human corneal innervation. Exp Eye Res. 2010;90:478–92. - PubMed
-
- Kitano S. An embryologic study of the human corneal nerves. Jap J Ophthalmol. 1957;1:48–55.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

