Therapeutic applications of dichloroacetate and the role of glutathione transferase zeta-1
- PMID: 27771434
- PMCID: PMC5274567
- DOI: 10.1016/j.pharmthera.2016.10.018
Therapeutic applications of dichloroacetate and the role of glutathione transferase zeta-1
Abstract
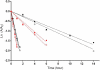
Dichloroacetate (DCA) has several therapeutic applications based on its pharmacological property of inhibiting pyruvate dehydrogenase kinase. DCA has been used to treat inherited mitochondrial disorders that result in lactic acidosis, as well as pulmonary hypertension and several different solid tumors, the latter through its ability to reverse the Warburg effect in cancer cells and restore aerobic glycolysis. The main clinically limiting toxicity is reversible peripheral neuropathy. Although administration of high doses to rodents can result in liver cancer, there is no evidence that DCA is a human carcinogen. In all studied species, including humans, DCA has the interesting property of inhibiting its own metabolism upon repeat dosing, resulting in alteration of its pharmacokinetics. The first step in DCA metabolism is conversion to glyoxylate catalyzed by glutathione transferase zeta 1 (GSTZ1), for which DCA is a mechanism-based inactivator. The rate of GSTZ1 inactivation by DCA is influenced by age, GSTZ1 haplotype and cellular concentrations of chloride. The effect of DCA on its own metabolism complicates the selection of an effective dose with minimal side effects.
Keywords: Dichloroacetate; GSTZ1; Inhibition of metabolism; Pharmacogenetics; Pharmacokinetics; Pyruvate dehydrogenase kinase.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
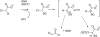
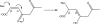


Similar articles
-
Exposure of Rats to Multiple Oral Doses of Dichloroacetate Results in Upregulation of Hepatic Glutathione Transferases and NAD(P)H Dehydrogenase [Quinone] 1.Drug Metab Dispos. 2020 Nov;48(11):1224-1230. doi: 10.1124/dmd.120.000143. Epub 2020 Sep 1. Drug Metab Dispos. 2020. PMID: 32873592 Free PMC article.
-
Pharmacogenetic considerations with dichloroacetate dosing.Pharmacogenomics. 2016 May;17(7):743-53. doi: 10.2217/pgs-2015-0012. Epub 2016 May 4. Pharmacogenomics. 2016. PMID: 27143230 Free PMC article. Review.
-
Effects of Multiple Doses of Dichloroacetate on GSTZ1 Expression and Activity in Liver and Extrahepatic Tissues of Young and Adult Rats.Drug Metab Dispos. 2020 Nov;48(11):1217-1223. doi: 10.1124/dmd.120.000142. Epub 2020 Sep 1. Drug Metab Dispos. 2020. PMID: 32873593 Free PMC article.
-
Chloride and other anions inhibit dichloroacetate-induced inactivation of human liver GSTZ1 in a haplotype-dependent manner.Chem Biol Interact. 2014 May 25;215:33-9. doi: 10.1016/j.cbi.2014.02.015. Epub 2014 Mar 13. Chem Biol Interact. 2014. PMID: 24632415 Free PMC article.
-
Role of dichloroacetate in the treatment of genetic mitochondrial diseases.Adv Drug Deliv Rev. 2008 Oct-Nov;60(13-14):1478-87. doi: 10.1016/j.addr.2008.02.014. Epub 2008 Jul 4. Adv Drug Deliv Rev. 2008. PMID: 18647626 Free PMC article. Review.
Cited by
-
Paraneoplastic syndrome in malignant lymphoma: A case report.Heliyon. 2023 Aug 15;9(8):e18968. doi: 10.1016/j.heliyon.2023.e18968. eCollection 2023 Aug. Heliyon. 2023. PMID: 37636455 Free PMC article.
-
Targeting Glucose Metabolism of Cancer Cells with Dichloroacetate to Radiosensitize High-Grade Gliomas.Int J Mol Sci. 2021 Jul 6;22(14):7265. doi: 10.3390/ijms22147265. Int J Mol Sci. 2021. PMID: 34298883 Free PMC article. Review.
-
Mitochondrial targeting by dichloroacetate improves outcome following hemorrhagic shock.Sci Rep. 2017 Jun 1;7(1):2671. doi: 10.1038/s41598-017-02495-5. Sci Rep. 2017. PMID: 28572638 Free PMC article.
-
Persistent gene expression and DNA methylation alterations linked to carcinogenic effects of dichloroacetic acid.Front Oncol. 2024 May 3;14:1389634. doi: 10.3389/fonc.2024.1389634. eCollection 2024. Front Oncol. 2024. PMID: 38764585 Free PMC article.
-
Exposure of Rats to Multiple Oral Doses of Dichloroacetate Results in Upregulation of Hepatic Glutathione Transferases and NAD(P)H Dehydrogenase [Quinone] 1.Drug Metab Dispos. 2020 Nov;48(11):1224-1230. doi: 10.1124/dmd.120.000143. Epub 2020 Sep 1. Drug Metab Dispos. 2020. PMID: 32873592 Free PMC article.
References
-
- Ammini CV, Fernandez-Canon J, Shroads AL, Cornett R, Cheung J, James MO, Henderson GN, Grompe M, Stacpoole PW. Pharmacologic or genetic ablation of maleylacetoacetate isomerase increases levels of toxic tyrosine catabolites in rodents. Biochem Pharmacol. 2003;66:2029–2038. - PubMed
-
- Anderson WB, Board PG, Gargano B, Anders MW. Inactivation of glutathione transferase zeta by dichloroacetic acid and other fluorine-lacking alpha-haloalkanoic acids. Chem Res Toxicol. 1999;12:1144–1149. - PubMed
-
- Anderson WB, Liebler DC, Board PG, Anders MW. Mass spectral characterization of dichloroacetic acid-modified human glutathione transferase zeta. Chem Res Toxicol. 2002;15:1387–1397. - PubMed
-
- Andonova IE, Justenhoven C, Winter S, Hamann U, Baisch C, Rabstein S, Spickenheuer A, Harth V, Pesch B, Bruning T, Ko YD, Ganev V, Brauch H. No evidence for glutathione S-transferases GSTA2, GSTM2, GSTO1, GSTO2, and GSTZ1 in breast cancer risk. Breast Cancer Res Treat. 2010;121:497–502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

