EphrinB3 restricts endogenous neural stem cell migration after traumatic brain injury
- PMID: 27771498
- PMCID: PMC5512275
- DOI: 10.1016/j.scr.2016.09.029
EphrinB3 restricts endogenous neural stem cell migration after traumatic brain injury
Abstract
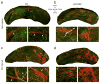
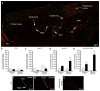
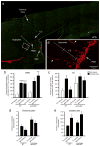
Traumatic brain injury (TBI) leads to a series of pathological events that can have profound influences on motor, sensory and cognitive functions. Conversely, TBI can also stimulate neural stem/progenitor cell proliferation leading to increased numbers of neuroblasts migrating outside their restrictive neurogenic zone to areas of damage in support of tissue integrity. Unfortunately, the factors that regulate migration are poorly understood. Here, we examine whether ephrinB3 functions to restrict neuroblasts from migrating outside the subventricular zone (SVZ) and rostral migratory stream (RMS). We have previously shown that ephrinB3 is expressed in tissues surrounding these regions, including the overlying corpus callosum (CC), and is reduced after controlled cortical impact (CCI) injury. Our current study takes advantage of ephrinB3 knockout mice to examine the influences of ephrinB3 on neuroblast migration into CC and cortex tissues after CCI injury. Both injury and/or ephrinB3 deficiency led to increased neuroblast numbers and enhanced migration outside the SVZ/RMS zones. Application of soluble ephrinB3-Fc molecules reduced neuroblast migration into the CC after injury and limited neuroblast chain migration in cultured SVZ explants. Our findings suggest that ephrinB3 expression in tissues surrounding neurogenic regions functions to restrict neuroblast migration outside the RMS by limiting chain migration.
Keywords: EphrinB3; Migration; Neuroblast; Traumatic brain injury.
Copyright © 2016 Michael Boutros, German Cancer Research Center, Heidelberg, Germany. Published by Elsevier B.V. All rights reserved.
Figures


Similar articles
-
EphB3 limits the expansion of neural progenitor cells in the subventricular zone by regulating p53 during homeostasis and following traumatic brain injury.Stem Cells. 2010 Jul;28(7):1231-42. doi: 10.1002/stem.449. Stem Cells. 2010. PMID: 20496368 Free PMC article.
-
Pronounced hypoxia in the subventricular zone following traumatic brain injury and the neural stem/progenitor cell response.Exp Biol Med (Maywood). 2013 Jul;238(7):830-41. doi: 10.1177/1535370213494558. Epub 2013 Jul 4. Exp Biol Med (Maywood). 2013. PMID: 23828590 Free PMC article.
-
A new function for Prokineticin 2: Recruitment of SVZ-derived neuroblasts to the injured cortex in a mouse model of traumatic brain injury.Mol Cell Neurosci. 2019 Jan;94:1-10. doi: 10.1016/j.mcn.2018.10.004. Epub 2018 Nov 1. Mol Cell Neurosci. 2019. PMID: 30391355
-
Research Advances in Neuroblast Migration in Traumatic Brain Injury.Mol Neurobiol. 2024 Oct;61(10):1-13. doi: 10.1007/s12035-024-04117-4. Epub 2024 Mar 20. Mol Neurobiol. 2024. PMID: 38507029 Free PMC article. Review.
-
Extracellular signals controlling neuroblast migration in the postnatal brain.Adv Exp Med Biol. 2014;800:149-80. doi: 10.1007/978-94-007-7687-6_9. Adv Exp Med Biol. 2014. PMID: 24243105 Review.
Cited by
-
Selective inhibition of soluble TNF using XPro1595 relieves pain and attenuates cerulein-induced pathology in mice.BMC Gastroenterol. 2021 May 28;21(1):243. doi: 10.1186/s12876-021-01827-0. BMC Gastroenterol. 2021. PMID: 34049483 Free PMC article.
-
DCC/netrin-1 regulates cell death in oligodendrocytes after brain injury.Cell Death Differ. 2023 Feb;30(2):397-406. doi: 10.1038/s41418-022-01091-z. Epub 2022 Dec 1. Cell Death Differ. 2023. PMID: 36456775 Free PMC article.
-
Roles of Astrocytic Endothelin ETB Receptor in Traumatic Brain Injury.Cells. 2023 Feb 24;12(5):719. doi: 10.3390/cells12050719. Cells. 2023. PMID: 36899860 Free PMC article. Review.
-
Explant Methodology for Analyzing Neuroblast Migration.Bio Protoc. 2017 May 5;7(9):e2249. doi: 10.21769/BioProtoc.2249. Bio Protoc. 2017. PMID: 28725659 Free PMC article.
-
The roles, mechanism, and mobilization strategy of endogenous neural stem cells in brain injury.Front Aging Neurosci. 2022 Aug 17;14:924262. doi: 10.3389/fnagi.2022.924262. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36062152 Free PMC article. Review.
References
-
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. - PubMed
-
- Bonfanti L, Theodosis DT. Expression of polysialylated neural cell adhesion molecule by proliferating cells in the subependymal layer of the adult rat, in its rostral extension and in the olfactory bulb. Neuroscience. 1994;62:291–305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

