Reduced vascular amyloid burden at microhemorrhage sites in cerebral amyloid angiopathy
- PMID: 27771772
- PMCID: PMC5325834
- DOI: 10.1007/s00401-016-1635-0
Reduced vascular amyloid burden at microhemorrhage sites in cerebral amyloid angiopathy
Abstract
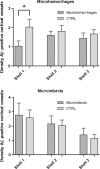
Microhemorrhages are strongly associated with advanced cerebral amyloid angiopathy (CAA). Although it has been frequently proposed that the deposition of Aβ in the walls of cortical vessels directly causes microhemorrhages, this has not been studied in great detail, mainly because the ruptured vessels are often missed on routine histopathologic examination. Here, we examined histopathological data from studies targeting microhemorrhages with high-resolution ex vivo 7 T MRI in nine cases with moderate-to-severe CAA, and assessed the presence of Aβ in the walls of involved vessels. We also assessed the density of Aβ positive cortical vessels in areas surrounding microhemorrhages compared to control areas. In seven out of 19 microhemorrhages, the presumed involved vessel could be identified on the histopathological section. Only one of these vessels was positive for Aβ at the site of rupture. Moreover, the density of Aβ positive cortical vessels was lower (1.0 per mm2) within a range of 315 µm surrounding the microhemorrhage, compared to control areas (2.0 per mm2; p < 0.05). These findings question the widely held assumption that the deposition of Aβ in the walls of cortical vessels directly causes microhemorrhages.
Keywords: Amyloid β; MRI; Microbleeds; Small vessel disease.
Conflict of interest statement
Funding
This work was supported by an Alzheimer Nederland fellowship [WE 15-2013-07], a Van Leersum grant of the Royal Dutch Academy of Sciences [2467-VLB-519], and a Rubicon Grant from the Netherlands Organization for Scientific Research [019.153LW.014] to SJvV, a VIDI Grant from ZonMw, The Netherlands Organization for Health Research and Development [91711384] to GJB, NIH Grants [R01AG047975], [P50AG005134], and [K23AG028726] to AV, and an NIH Grant [R01AG26484] to AV and SMG. HJK was financially supported by the project Brainbox (Quantitative analysis of MR brain images for cerebrovascular disease management), funded by the Netherlands Organization for Health Research and Development (ZonMw) in the framework of the research program IMDI (Innovative Medical Devices Initiative); Project 104002002.
Conflict of interest
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
In vivo characterization of spontaneous microhemorrhage formation in mice with cerebral amyloid angiopathy.J Cereb Blood Flow Metab. 2021 Jan;41(1):82-91. doi: 10.1177/0271678X19899377. Epub 2020 Jan 27. J Cereb Blood Flow Metab. 2021. PMID: 31987010 Free PMC article.
-
Brain hemorrhages in cerebral amyloid angiopathy.Semin Thromb Hemost. 2013 Nov;39(8):955-62. doi: 10.1055/s-0033-1357489. Epub 2013 Oct 9. Semin Thromb Hemost. 2013. PMID: 24108472 Review.
-
Colocalization of different types of amyloid in the walls of cerebral blood vessels of patients suffering from cerebral amyloid angiopathy and spontaneous intracranial hemorrhage: a report of 5 cases.Clin Neuropathol. 2004 May-Jun;23(3):113-9. Clin Neuropathol. 2004. PMID: 15200289
-
Spatial relation between microbleeds and amyloid deposits in amyloid angiopathy.Ann Neurol. 2010 Oct;68(4):545-8. doi: 10.1002/ana.22099. Ann Neurol. 2010. PMID: 20865701 Free PMC article.
-
Cerebral microhemorrhage.Stroke. 2006 Feb;37(2):550-5. doi: 10.1161/01.STR.0000199847.96188.12. Epub 2006 Jan 5. Stroke. 2006. PMID: 16397165 Review.
Cited by
-
Disturbed balance in the expression of MMP9 and TIMP3 in cerebral amyloid angiopathy-related intracerebral haemorrhage.Acta Neuropathol Commun. 2020 Jul 6;8(1):99. doi: 10.1186/s40478-020-00972-z. Acta Neuropathol Commun. 2020. PMID: 32631441 Free PMC article.
-
In vivo characterization of spontaneous microhemorrhage formation in mice with cerebral amyloid angiopathy.J Cereb Blood Flow Metab. 2021 Jan;41(1):82-91. doi: 10.1177/0271678X19899377. Epub 2020 Jan 27. J Cereb Blood Flow Metab. 2021. PMID: 31987010 Free PMC article.
-
Vascular pathology and pathogenesis of cognitive impairment and dementia in older adults.Cereb Circ Cogn Behav. 2022 Jun 30;3:100148. doi: 10.1016/j.cccb.2022.100148. eCollection 2022. Cereb Circ Cogn Behav. 2022. PMID: 36324408 Free PMC article.
-
Association of amyloid angiopathy with microbleeds in logopenic progressive aphasia: an imaging-pathology study.Eur J Neurol. 2021 Feb;28(2):670-675. doi: 10.1111/ene.14594. Epub 2020 Nov 12. Eur J Neurol. 2021. PMID: 33068458 Free PMC article.
-
Age, sex, and cerebral microbleeds in EFAD Alzheimer disease mice.Neurobiol Aging. 2021 Jul;103:42-51. doi: 10.1016/j.neurobiolaging.2021.02.020. Epub 2021 Feb 28. Neurobiol Aging. 2021. PMID: 33813349 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

