Temporal, Diagnostic, and Tissue-Specific Regulation of NRG3 Isoform Expression in Human Brain Development and Affective Disorders
- PMID: 27771971
- PMCID: PMC5892449
- DOI: 10.1176/appi.ajp.2016.16060721
Temporal, Diagnostic, and Tissue-Specific Regulation of NRG3 Isoform Expression in Human Brain Development and Affective Disorders
Abstract
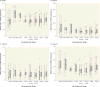
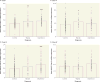
Objective: Genes implicated in schizophrenia are enriched in networks differentially regulated during human CNS development. Neuregulin 3 (NRG3), a brain-enriched neurotrophin, undergoes alternative splicing and is implicated in several neurological disorders with developmental origins. Isoform-specific increases in NRG3 are observed in schizophrenia and associated with rs10748842, a NRG3 risk polymorphism, suggesting NRG3 transcriptional dysregulation as a molecular mechanism of risk. The authors quantitatively mapped the temporal trajectories of NRG3 isoforms (classes I-IV) in the neocortex throughout the human lifespan, examined whether tissue-specific regulation of NRG3 occurs in humans, and determined if abnormalities in NRG3 transcriptomics occur in mood disorders and are genetically determined.
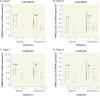
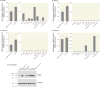
Method: NRG3 isoform classes I-IV were quantified using quantitative real-time polymerase chain reaction in human postmortem dorsolateral prefrontal cortex from 286 nonpsychiatric control individuals, from gestational week 14 to 85 years old, and individuals diagnosed with either bipolar disorder (N=34) or major depressive disorder (N=69). Tissue-specific mapping was investigated in several human tissues. rs10748842 was genotyped in individuals with mood disorders, and association with NRG3 isoform expression examined.
Results: NRG3 classes displayed individually specific expression trajectories across human neocortical development and aging; classes I, II, and IV were significantly associated with developmental stage. NRG3 class I was increased in bipolar and major depressive disorder, consistent with observations in schizophrenia. NRG3 class II was increased in bipolar disorder, and class III was increased in major depression. The rs10748842 risk genotype predicted elevated class II and III expression, consistent with previous reports in the brain, with tissue-specific analyses suggesting that classes II and III are brain-specific isoforms of NRG3.
Conclusions: Mapping the temporal expression of genes during human brain development provides vital insight into gene function and identifies critical sensitive periods whereby genetic factors may influence risk for psychiatric disease. Here the authors provide comprehensive insight into the transcriptional landscape of the psychiatric risk gene, NRG3, in human neocortical development and expand on previous findings in schizophrenia to identify increased expression of developmentally and genetically regulated isoforms in the brain of patients with mood disorders. Principally, the finding that NRG3 classes II and III are brain-specific isoforms predicted by rs10748842 risk genotype and are increased in mood disorders further implicates a molecular mechanism of psychiatric risk at the NRG3 locus and identifies a potential developmental role for NRG3 in bipolar disorder and major depression. These observations encourage investigation of the neurobiology of NRG3 isoforms and highlight inhibition of NRG3 signaling as a potential target for psychiatric treatment development.
Keywords: ErbB4; Genetics; Mood Disorders-Bipolar; Mood Disorders-Unipolar; Neuregulin; Schizophrenia.
Conflict of interest statement
The other authors report no financial relationships with commercial interests.
Figures
Similar articles
-
Common genetic variation in Neuregulin 3 (NRG3) influences risk for schizophrenia and impacts NRG3 expression in human brain.Proc Natl Acad Sci U S A. 2010 Aug 31;107(35):15619-24. doi: 10.1073/pnas.1005410107. Epub 2010 Aug 16. Proc Natl Acad Sci U S A. 2010. PMID: 20713722 Free PMC article.
-
Expression of ZNF804A in human brain and alterations in schizophrenia, bipolar disorder, and major depressive disorder: a novel transcript fetally regulated by the psychosis risk variant rs1344706.JAMA Psychiatry. 2014 Oct;71(10):1112-20. doi: 10.1001/jamapsychiatry.2014.1079. JAMA Psychiatry. 2014. PMID: 25162540 Free PMC article.
-
Transcript-specific associations of SLC12A5 (KCC2) in human prefrontal cortex with development, schizophrenia, and affective disorders.J Neurosci. 2012 Apr 11;32(15):5216-22. doi: 10.1523/JNEUROSCI.4626-11.2012. J Neurosci. 2012. PMID: 22496567 Free PMC article.
-
An overview of the genetics of psychotic mood disorders.J Psychiatr Res. 2004 Jan;38(1):3-15. doi: 10.1016/s0022-3956(03)00096-7. J Psychiatr Res. 2004. PMID: 14690766 Review.
-
In vivo magnetic resonance spectroscopy and its application to neuropsychiatric disorders.Can J Psychiatry. 2002 May;47(4):315-26. doi: 10.1177/070674370204700402. Can J Psychiatry. 2002. PMID: 12025430 Review.
Cited by
-
Characterisation and functional predictions of canine long non-coding RNAs.Sci Rep. 2018 Sep 7;8(1):13444. doi: 10.1038/s41598-018-31770-2. Sci Rep. 2018. PMID: 30194329 Free PMC article.
-
De novo truncating NOVA2 variants affect alternative splicing and lead to heterogeneous neurodevelopmental phenotypes.Hum Mutat. 2022 Sep;43(9):1299-1313. doi: 10.1002/humu.24414. Epub 2022 Jun 8. Hum Mutat. 2022. PMID: 35607920 Free PMC article.
-
Tracking cell-type-specific temporal dynamics in human and mouse brains.Cell. 2023 Sep 28;186(20):4345-4364.e24. doi: 10.1016/j.cell.2023.08.042. Cell. 2023. PMID: 37774676 Free PMC article.
-
Neuregulin 3 and its roles in schizophrenia risk and presentation.Am J Med Genet B Neuropsychiatr Genet. 2018 Mar;177(2):257-266. doi: 10.1002/ajmg.b.32552. Epub 2017 May 29. Am J Med Genet B Neuropsychiatr Genet. 2018. PMID: 28556469 Free PMC article. Review.
-
An alternative splicing hypothesis for neuropathology of schizophrenia: evidence from studies on historical candidate genes and multi-omics data.Mol Psychiatry. 2022 Jan;27(1):95-112. doi: 10.1038/s41380-021-01037-w. Epub 2021 Mar 8. Mol Psychiatry. 2022. PMID: 33686213 Review.
References
-
- Faraone SV, Hwu HG, Liu CM, et al. Genome scan of Han Chinese schizophrenia families from Taiwan: confirmation of linkage to 10q22.3. Am J Psychiatry. 2006;163:1760–1766. - PubMed
-
- Sonuga-Barke EJ, Lasky-Su J, Neale BM, et al. Does parental expressed emotion moderate genetic effects in ADHD? an exploration using a genome wide association scan. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1359–1368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

