DCC Confers Susceptibility to Depression-like Behaviors in Humans and Mice and Is Regulated by miR-218
- PMID: 27773352
- PMCID: PMC5239724
- DOI: 10.1016/j.biopsych.2016.08.017
DCC Confers Susceptibility to Depression-like Behaviors in Humans and Mice and Is Regulated by miR-218
Abstract
Backgroud: Variations in the expression of the Netrin-1 guidance cue receptor DCC (deleted in colorectal cancer) appear to confer resilience or susceptibility to psychopathologies involving prefrontal cortex (PFC) dysfunction.
Methods: With the use of postmortem brain tissue, mouse models of defeat stress, and in vitro analysis, we assessed microRNA (miRNA) regulation of DCC and whether changes in DCC levels in the PFC lead to vulnerability to depression-like behaviors.
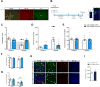
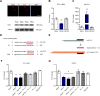
Results: We identified miR-218 as a posttranscriptional repressor of DCC and detected coexpression of DCC and miR-218 in pyramidal neurons of human and mouse PFC. We found that exaggerated expression of DCC and reduced levels of miR-218 in the PFC are consistent traits of mice susceptible to chronic stress and of major depressive disorder in humans. Remarkably, upregulation of Dcc in mouse PFC pyramidal neurons causes vulnerability to stress-induced social avoidance and anhedonia.
Conclusions: These data are the first demonstration of microRNA regulation of DCC and suggest that, by regulating DCC, miR-218 may be a switch of susceptibility versus resilience to stress-related disorders.
Keywords: Chronic social defeat stress; Guidance cue; Major depressive disorder; Neurodevelopment; Resilience; microRNA.
Copyright © 2016 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
All authors report no biomedical financial interests or potential conflicts of interest.
Figures


Similar articles
-
Relationship between insulin and Netrin-1/DCC guidance cue pathway regulation in the prefrontal cortex of rodents exposed to prenatal dietary restriction.J Dev Orig Health Dis. 2023 Aug;14(4):501-507. doi: 10.1017/S204017442300017X. Epub 2023 Jul 11. J Dev Orig Health Dis. 2023. PMID: 37431265 Free PMC article.
-
The Netrin-1/DCC Guidance Cue Pathway as a Molecular Target in Depression: Translational Evidence.Biol Psychiatry. 2020 Oct 15;88(8):611-624. doi: 10.1016/j.biopsych.2020.04.025. Epub 2020 May 11. Biol Psychiatry. 2020. PMID: 32593422 Free PMC article. Review.
-
miR-218 in Adolescence Predicts and Mediates Vulnerability to Stress.Biol Psychiatry. 2021 May 1;89(9):911-919. doi: 10.1016/j.biopsych.2020.10.015. Epub 2020 Nov 2. Biol Psychiatry. 2021. PMID: 33384174 Free PMC article.
-
The netrin receptor DCC is required in the pubertal organization of mesocortical dopamine circuitry.J Neurosci. 2011 Jun 8;31(23):8381-94. doi: 10.1523/JNEUROSCI.0606-11.2011. J Neurosci. 2011. PMID: 21653843 Free PMC article.
-
Awakening the dormant: Role of axonal guidance cues in stress-induced reorganization of the adult prefrontal cortex leading to depression-like behavior.Front Neural Circuits. 2023 Mar 24;17:1113023. doi: 10.3389/fncir.2023.1113023. eCollection 2023. Front Neural Circuits. 2023. PMID: 37035502 Free PMC article. Review.
Cited by
-
Epigenetic mechanisms impacted by chronic stress across the rodent lifespan.Neurobiol Stress. 2022 Jan 31;17:100434. doi: 10.1016/j.ynstr.2022.100434. eCollection 2022 Mar. Neurobiol Stress. 2022. PMID: 35198660 Free PMC article.
-
MicroRNAs in depression and suicide: Recent insights and future perspectives.J Affect Disord. 2018 Nov;240:146-154. doi: 10.1016/j.jad.2018.07.075. Epub 2018 Jul 24. J Affect Disord. 2018. PMID: 30071418 Free PMC article. Review.
-
MicroRNAs in the Onset of Schizophrenia.Cells. 2021 Oct 6;10(10):2679. doi: 10.3390/cells10102679. Cells. 2021. PMID: 34685659 Free PMC article. Review.
-
Epigenetic regulation in major depression and other stress-related disorders: molecular mechanisms, clinical relevance and therapeutic potential.Signal Transduct Target Ther. 2023 Aug 30;8(1):309. doi: 10.1038/s41392-023-01519-z. Signal Transduct Target Ther. 2023. PMID: 37644009 Free PMC article. Review.
-
Convergence of evidence from a methylome-wide CpG-SNP association study and GWAS of major depressive disorder.Transl Psychiatry. 2018 Aug 22;8(1):162. doi: 10.1038/s41398-018-0205-8. Transl Psychiatry. 2018. PMID: 30135428 Free PMC article.
References
-
- Moore SW, Tessier-Lavigne M, Kennedy TE. Netrins and Their receptors. Adv Exp Med Biol. 2007;621:17–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

