Circadian Clock Interaction with HIF1α Mediates Oxygenic Metabolism and Anaerobic Glycolysis in Skeletal Muscle
- PMID: 27773696
- PMCID: PMC5226863
- DOI: 10.1016/j.cmet.2016.09.010
Circadian Clock Interaction with HIF1α Mediates Oxygenic Metabolism and Anaerobic Glycolysis in Skeletal Muscle
Abstract
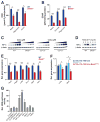
Circadian clocks are encoded by a transcription-translation feedback loop that aligns energetic processes with the solar cycle. We show that genetic disruption of the clock activator BMAL1 in skeletal myotubes and fibroblasts increased levels of the hypoxia-inducible factor 1α (HIF1α) under hypoxic conditions. Bmal1-/- myotubes displayed reduced anaerobic glycolysis, mitochondrial respiration with glycolytic fuel, and transcription of HIF1α targets Phd3, Vegfa, Mct4, Pk-m, and Ldha, whereas abrogation of the clock repressors CRY1/2 stabilized HIF1α in response to hypoxia. HIF1α bound directly to core clock gene promoters, and, when co-expressed with BMAL1, led to transactivation of PER2-LUC and HRE-LUC reporters. Further, genetic stabilization of HIF1α in Vhl-/- cells altered circadian transcription. Finally, induction of clock and HIF1α target genes in response to strenuous exercise varied according to the time of day in wild-type mice. Collectively, our results reveal bidirectional interactions between circadian and HIF pathways that influence metabolic adaptation to hypoxia.
Keywords: BMAL1; HIF; circadian; clock; exercise; glycolysis; hypoxia; lactate; mitochondria; skeletal muscle.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures

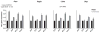
Comment in
-
Daily oxygen rhythms.Sci Signal. 2017 Jan 31;10(464):eaam8695. doi: 10.1126/scisignal.aam8695. Sci Signal. 2017. PMID: 28143912
References
-
- Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. - PubMed
-
- Bass J. Circadian topology of metabolism. Nature. 2012;491:348–356. - PubMed
-
- Bersten DC, Sullivan AE, Peet DJ, Whitelaw ML. bHLH-PAS proteins in cancer. Nature reviews Cancer. 2013;13:827–841. - PubMed
-
- Chilov D, Camenisch G, Kvietikova I, Ziegler U, Gassmann M, Wenger RH. Induction and nuclear translocation of hypoxia-inducible factor-1 (HIF-1): heterodimerization with ARNT is not necessary for nuclear accumulation of HIF-1alpha. J Cell Sci. 1999;112(Pt 8):1203–1212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

