Partial MHC class II constructs as novel immunomodulatory therapy for stroke
- PMID: 27773790
- PMCID: PMC5411346
- DOI: 10.1016/j.neuint.2016.10.007
Partial MHC class II constructs as novel immunomodulatory therapy for stroke
Abstract
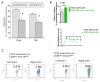
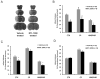
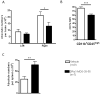
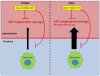
The worldwide prevalence of stroke continues to rise despite recent successes in treating acute ischemic stroke. With limited patient eligibility and associated risk of tPA and mechanical thrombectomy, new preventive and therapeutic modalities are needed to stave the rising wave of stroke. Inflammation plays a key role in brain damage after cerebral ischemia, and novel therapies that target pro-inflammatory cells have demonstrated promise for treatment for stroke. Partial MHC class II constructs have been shown to prevent and/or reverse clinical signs of various inflammatory diseases such as experimental autoimmune encephalomyelitis, collagen-induced arthritis and experimental autoimmune uveitis, by reducing the number and frequency of activated cells in the damaged CNS. Herein, we review the use of partial MHC class II constructs as a novel treatment for ischemic stroke. These constructs have been shown to reduce infarct volume and neurological deficit in various cerebral ischemia models in young adult and aging male and female mice. In addition, partial MHC class II constructs were shown to reverse stroke-associated splenic atrophy and promote a protective M2 macrophage/microglia phenotype in the CNS which contributes to tissue repair and recovery after stroke. By addressing remaining STAIR criteria, such as efficacy in large animal models of stroke, these constructs will be prime candidates for clinical trials of acute ischemic stroke.
Keywords: Immunotherapy; Inflammation; Partial MHC class II construct; Recombinant T-cell receptor ligand; Stroke.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Drs. Vandenbark, Offner, Benedek, Alkayed and OHSU have a significant financial interest in Artielle ImmunoTherapeutics, Inc., a company that may have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by the OHSU and VAPHCS Conflict of Interest in Research Committees.
Figures
Similar articles
-
Spleen participation in partial MHC class II construct neuroprotection in stroke.CNS Neurosci Ther. 2020 Jul;26(7):663-669. doi: 10.1111/cns.13369. Epub 2020 Mar 31. CNS Neurosci Ther. 2020. PMID: 32237074 Free PMC article. Review.
-
Partial MHC Constructs Treat Thromboembolic Ischemic Stroke Characterized by Early Immune Expansion.Transl Stroke Res. 2016 Feb;7(1):70-8. doi: 10.1007/s12975-015-0436-4. Epub 2015 Dec 1. Transl Stroke Res. 2016. PMID: 26627498 Free PMC article.
-
Upregulation of CD74 and its potential association with disease severity in subjects with ischemic stroke.Neurochem Int. 2017 Jul;107:148-155. doi: 10.1016/j.neuint.2016.11.007. Epub 2016 Nov 21. Neurochem Int. 2017. PMID: 27884769 Free PMC article.
-
HLA-DRα1-mMOG-35-55 treatment of experimental autoimmune encephalomyelitis reduces CNS inflammation, enhances M2 macrophage frequency, and promotes neuroprotection.J Neuroinflammation. 2015 Jun 24;12:123. doi: 10.1186/s12974-015-0342-4. J Neuroinflammation. 2015. PMID: 26104759 Free PMC article.
-
Human major histocompatibility complex. Genes and diseases.J Fla Med Assoc. 1992 Feb;79(2):97-9. J Fla Med Assoc. 1992. PMID: 1552300 Review.
Cited by
-
T Cell Response in Ischemic Stroke: From Mechanisms to Translational Insights.Front Immunol. 2021 Jul 15;12:707972. doi: 10.3389/fimmu.2021.707972. eCollection 2021. Front Immunol. 2021. PMID: 34335623 Free PMC article. Review.
-
DRα1-MOG-35-55 treatment reduces lesion volumes and improves neurological deficits after traumatic brain injury.Metab Brain Dis. 2017 Oct;32(5):1395-1402. doi: 10.1007/s11011-017-9991-6. Epub 2017 Mar 16. Metab Brain Dis. 2017. PMID: 28303450 Free PMC article.
-
Microglia and Macrophages in Neuroprotection, Neurogenesis, and Emerging Therapies for Stroke.Cells. 2021 Dec 16;10(12):3555. doi: 10.3390/cells10123555. Cells. 2021. PMID: 34944064 Free PMC article. Review.
-
Major histocompatibility complex Class II-based therapy for stroke.Brain Circ. 2021 Mar 30;7(1):37-40. doi: 10.4103/bc.bc_16_21. eCollection 2021 Jan-Mar. Brain Circ. 2021. PMID: 34084976 Free PMC article. Review.
-
Novel therapeutic for multiple sclerosis protects white matter function in EAE mouse model.Front Mol Med. 2023;3:1237078. doi: 10.3389/fmmed.2023.1237078. Epub 2023 Aug 28. Front Mol Med. 2023. PMID: 37933270 Free PMC article.
References
-
- Adamus G, Burrows GG, Vandenbark AA, Offner H. Treatment of autoimmune anterior uveitis with recombinant TCR ligands. Invest Ophthalmol Vis Sci. 2006;47:2555–2561. - PubMed
-
- Akopov SE, Simonian NA, Grigorian GS. Dynamics of polymorphonuclear leukocyte accumulation in acute cerebral infarction and their correlation with brain tissue damage. Stroke. 1996;27:1739–1743. - PubMed
-
- Albers GW, Bates VE, Clark WM, Bell R, Verro P, Hamilton SA. Intravenous tissue-type plasminogen activator for treatment of acute stroke: the Standard Treatment with Alteplase to Reverse Stroke (STARS) study. JAMA. 2000;283:1145–1150. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

