Residual Isocyanates in Medical Devices and Products: A Qualitative and Quantitative Assessment
- PMID: 27773989
- PMCID: PMC5067089
- DOI: 10.4137/EHI.S39149
Residual Isocyanates in Medical Devices and Products: A Qualitative and Quantitative Assessment
Abstract
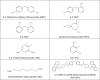
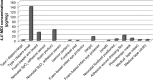
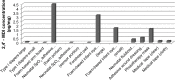
We conducted a pilot qualitative and quantitative assessment of residual isocyanates and their potential initial exposures in neonates, as little is known about their contact effect. After a neonatal intensive care unit (NICU) stockroom inventory, polyurethane (PU) and PU foam (PUF) devices and products were qualitatively evaluated for residual isocyanates using Surface SWYPE™. Those containing isocyanates were quantitatively tested for methylene diphenyl diisocyanate (MDI) species, using UPLC-UV-MS/MS method. Ten of 37 products and devices tested, indicated both free and bound residual surface isocyanates; PU/PUF pieces contained aromatic isocyanates; one product contained aliphatic isocyanates. Overall, quantified mean MDI concentrations were low (4,4'-MDI = 0.52 to 140.1 pg/mg) and (2,4'-MDI = 0.01 to 4.48 pg/mg). The 4,4'-MDI species had the highest measured concentration (280 pg/mg). Commonly used medical devices/products contain low, but measurable concentrations of residual isocyanates. Quantifying other isocyanate species and neonatal skin exposure to isocyanates from these devices and products requires further investigation.
Keywords: asthma; isocyanates; medical devices and products; methylene diphenyl diisocyanate; neonatal exposure; skin sensitization.
Figures
References
-
- Mannino DM, Homa DM, Pertowski CA, Ashizawa A, Nixon LL, Johnson CA, et al. Surveillance for asthma–United States, 1960–1995. MMWR CDC Surveill Summ. 1998;47(1):1–27. - PubMed
-
- Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma–United States, 1980–1999. MMWR Surveill Summ. 2002;51(1):1–13. - PubMed
-
- Akinbami L, the Centers for Disease Control and Prevention National Center for Health Statistics The state of childhood asthma, United States, 1980–2005. Adv Data. 2006;12(381):1–24. - PubMed
-
- Lin RY, Pitt TJ, Lou WY, Yi Q. Asthma hospitalization patterns in young children relating to admission age, infection presence, sex, and race. Ann Allergy Asthma Immunol. 2007;98(2):139–45. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources