Multiple Effects of Growth Hormone in the Body: Is it Really the Hormone for Growth?
- PMID: 27773998
- PMCID: PMC5063841
- DOI: 10.4137/CMED.S38201
Multiple Effects of Growth Hormone in the Body: Is it Really the Hormone for Growth?
Abstract
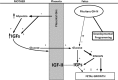
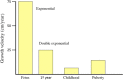
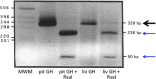
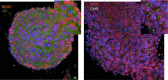
In this review, we analyze the effects of growth hormone on a number of tissues and organs and its putative role in the longitudinal growth of an organism. We conclude that the hormone plays a very important role in maintaining the homogeneity of tissues and organs during the normal development of the human body or after an injury. Its effects on growth do not seem to take place during the fetal period or during the early infancy and are mediated by insulin-like growth factor I (IGF-I) during childhood and puberty. In turn, IGF-I transcription is dependent on an adequate GH secretion, and in many tissues, it occurs independent of GH. We propose that GH may be a prohormone, rather than a hormone, since in many tissues and organs, it is proteolytically cleaved in a tissue-specific manner giving origin to shorter GH forms whose activity is still unknown.
Keywords: cardiovascular system; gonads; growth; growth hormone; liver; nervous system.
Conflict of interest statement
Authors disclose no potential conflicts of interest.
Figures

References
-
- Evans HM, Long JA. The effect of the anterior lobe administered intraperitoneally upon growth maturation, and oestrus cycles of the rat. Anatomical Record. 1921;21:62–63.
-
- Raben MS. Treatment of a pituitary dwarf with human growth hormone. J Clin Endocrinol Metab. 1958;18:901–903. - PubMed
-
- Keohane C. Prion diseases in men. Arch Anat Cytol Pathol. 1994;42:69–75. - PubMed
-
- Stockdale T. Contaminated material caused Creutzfeld-Jakob disease (CJD) in some undersized children who were treated with growth hormone (GH) Nutr Health. 2000;14:141–142. - PubMed
-
- Sairam MR, Chrétien M, Li CH. On the isolation of human pituitary hormones. J Clin Endocrinol Metab. 1978;47:1002–1008. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

