Initial 3-year outcomes with left ventricular assist devices in a country with a nascent heart transplantation program
- PMID: 27774264
- PMCID: PMC5061086
- DOI: 10.1002/ehf2.12066
Initial 3-year outcomes with left ventricular assist devices in a country with a nascent heart transplantation program
Abstract
Aims: The need for the left ventricular assist devices (LVAD) in patients with end-stage heart failure is well established, but prior to 2011, this was not available to patients in Kazakhstan. We describe the development of the sole LVAD programme in the context of a nascent heart transplantation programme and clinical outcomes for the first three years.
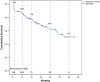
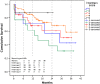
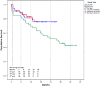
Methods and results: From November 2011 to November 2014, 146 patients underwent implantation of 152 VADs (approximately 50 devices implanted per year). We retrospectively analyzed data from 135 LVAD patients who received HeartMate II (n = 95) or HeartWare (n = 40) devices. In 75 patients LVAD was used as a bridge-to-transplantation and in 60 patients as destination therapy, but only 3 of 135 LVAD patients received heart transplant. Forty-three patients of the LVAD cohort had died by the end of the follow-up period. The mean time on LVAD was 466 ± 330 days (range 5-1200 days). Kaplan-Meier survival estimates for patients who continued on LVAD support were 93% after 1 month, 86% after 6 months and 77% after 12 months. The most common complications within the first 30 days after implant included right ventricular failure (n = 20, 1.85 events/patient-year), renal failure (n = 19, 1.76 events/patient-year) and bleeding (n = 33, 3.0 events/patient-year). Beyond 30 days adverse events included driveline infections (n = 46, 0.56 events/patient-year) and stroke (n = 33, 0.21 events/patient-year).
Conclusions: LVADs are an important therapeutic alternative to heart transplantation in the context of a developing heart transplant programme with outcomes that are comparable to those reported by other centres.
Keywords: Heart failure; Heart transplantation; Kazakhstan; Left ventricular assist device; Mechanical circulatory support.
Figures
References
-
- Starling RC. Improved quantity and quality of life: a winning combination to treat advanced heart failure. J Am Coll Cardiol 2010; 55: 1835–1836. - PubMed
-
- Starling RC, Naka Y, Boyle AJ, Gonzalez‐Stawinski G, John R, Jorde U, Russel SD, Conte JV, Aaronson KD, McGee EC Jr, Cotts WG, DeNofrio D, Thinh Pham D, Farrar DJ, Pagani FD. Results of the post‐U.S. Food and Drug Administration‐approval study with a continuous‐flow left ventricular assist device as a bridge to heart transplantation: a prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support). J Am Coll Cardiol 2011; 57: 1890–1898. - PubMed
-
- John R, Naka Y, Smedira NG, Starling R, Jorde U, Eckman P, Farrar DJ, Pagani FD. Continuous‐flow left ventricular assist device outcomes in commercial use compared with the prior clinical trial. Ann Thorac Surg 2011; 92: 1406–1413; discussion 1413. - PubMed
-
- John R, Kamdar F, Liao K, Colvin‐Adams M, Boyle A, Joyce L. Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge‐to‐transplant therapy. Ann Thorac Surg 2008; 86: 1227–1234; discussion 1234–1225. - PubMed
-
- Maciver J, Ross HJ. Quality of life and left ventricular assist device support. Circulation 2012; 126: 866–874. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

