Experimental Models of Foamy Macrophages and Approaches for Dissecting the Mechanisms of Lipid Accumulation and Consumption during Dormancy and Reactivation of Tuberculosis
- PMID: 27774438
- PMCID: PMC5054039
- DOI: 10.3389/fcimb.2016.00122
Experimental Models of Foamy Macrophages and Approaches for Dissecting the Mechanisms of Lipid Accumulation and Consumption during Dormancy and Reactivation of Tuberculosis
Abstract
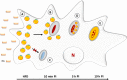
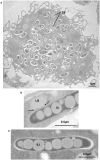
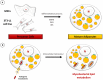
Despite a slight decline since 2014, tuberculosis (TB) remains the major deadly infectious disease worldwide with about 1.5 million deaths each year and with about one-third of the population being latently infected with Mycobacterium tuberculosis, the etiologic agent of TB. During primo-infection, the recruitment of immune cells leads to the formation of highly organized granulomas. Among the different cells, one outstanding subpopulation is the foamy macrophage (FM), characterized by the abundance of triacylglycerol-rich lipid bodies (LB). M. tuberculosis can reside in FM, where it acquires, from host LB, the neutral lipids which are subsequently processed and stored by the bacilli in the form of intracytosolic lipid inclusions (ILI). Although host LB can be viewed as a reservoir of nutrients for the pathogen during latency, the molecular mechanisms whereby intraphagosomal mycobacteria interact with LB and assimilate the LB-derived lipids are only beginning to be understood. Past studies have emphasized that these physiological processes are critical to the M. tuberculosis infectious-life cycle, for propagation of the infection, establishment of the dormancy state and reactivation of the disease. In recent years, several animal and cellular models have been developed with the aim of dissecting these complex processes and of determining the nature and contribution of their key players. Herein, we review some of the in vitro and in vivo models which allowed to gain significant insight into lipid accumulation and consumption in M. tuberculosis, two important events that are directly linked to pathogenicity, granuloma formation/maintenance and survival of the tubercle bacillus under non-replicative conditions. We also discuss the advantages and limitations of each model, hoping that this will serve as a guide for future investigations dedicated to persistence and innovative therapeutic approaches against TB.
Keywords: adipocyte; amoeba; granuloma; lipid body; mycobacteria; pathogenesis.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

