Hypoxia-inducible factor-mediated induction of WISP-2 contributes to attenuated progression of breast cancer
- PMID: 27774464
- PMCID: PMC5045054
- DOI: 10.2147/HP.S54404
Hypoxia-inducible factor-mediated induction of WISP-2 contributes to attenuated progression of breast cancer
Abstract
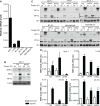
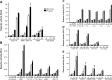
Hypoxia and the hypoxia-inducible factor (HIF) signaling pathway trigger the expression of several genes involved in cancer progression and resistance to therapy. Transcriptionally active HIF-1 and HIF-2 regulate overlapping sets of target genes, and only few HIF-2 specific target genes are known so far. Here we investigated oxygen-regulated expression of Wnt-1 induced signaling protein 2 (WISP-2), which has been reported to attenuate the progression of breast cancer. WISP-2 was hypoxically induced in low-invasive luminal-like breast cancer cell lines at both the messenger RNA and protein levels, mainly in a HIF-2α-dependent manner. HIF-2-driven regulation of the WISP2 promoter in breast cancer cells is almost entirely mediated by two phylogenetically and only partially conserved functional hypoxia response elements located in a microsatellite region upstream of the transcriptional start site. High WISP-2 tumor levels were associated with increased HIF-2α, decreased tumor macrophage density, and a better prognosis. Silencing WISP-2 increased anchorage-independent colony formation and recovery from scratches in confluent cell layers of normally low-invasive MCF-7 cancer cells. Interestingly, these changes in cancer cell aggressiveness could be phenocopied by HIF-2α silencing, suggesting that direct HIF-2-mediated transcriptional induction of WISP-2 gene expression might at least partially explain the association of high HIF-2α tumor levels with prolonged overall survival of patients with breast cancer.
Keywords: invasion; metastasis; motility; oxygen; transcriptional regulation; tumor.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

