The impact of hypoxia in pancreatic cancer invasion and metastasis
- PMID: 27774469
- PMCID: PMC5045059
- DOI: 10.2147/HP.S52636
The impact of hypoxia in pancreatic cancer invasion and metastasis
Abstract
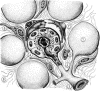
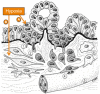
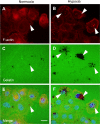
Intratumoral hypoxia is a common feature of solid tumors. Recent advances in cancer biology indicate that hypoxia is not only a consequence of unrestrained tumor growth, but also plays an active role in promoting tumor progression, malignancy, and resistance to therapy. Hypoxia signaling is mediated by the hypoxia-inducible factors (HIFs), which are not only stabilized under hypoxia, but also by activated oncogenes or inactivated tumor suppressors under normoxia. Hypoxia is a prominent feature of the tumor microenvironment of pancreatic tumors, also characterized by the presence of a fibrotic reaction that promotes, and is also modulated by, hypoxia. As the mechanisms by which hypoxia signaling impacts invasion and metastasis in pancreatic cancer are being elucidated, hypoxia is emerging as a key determinant of pancreatic cancer malignancy as well as an important target for therapy. Herein we present an overview of recent advances in the understanding of the impact that hypoxia has in pancreatic cancer invasion and metastasis.
Keywords: EMT; HIF-1; PDAC; PanIN; invadopodia; invasion.
Figures


Similar articles
-
Correlation between hypoxia and HGF/c-MET expression in the management of pancreatic cancer.Biochim Biophys Acta Rev Cancer. 2023 May;1878(3):188869. doi: 10.1016/j.bbcan.2023.188869. Epub 2023 Feb 24. Biochim Biophys Acta Rev Cancer. 2023. PMID: 36842767 Review.
-
Hypoxia-inducible factor-1 promotes pancreatic ductal adenocarcinoma invasion and metastasis by activating transcription of the actin-bundling protein fascin.Cancer Res. 2014 May 1;74(9):2455-64. doi: 10.1158/0008-5472.CAN-13-3009. Epub 2014 Mar 5. Cancer Res. 2014. PMID: 24599125
-
Effects of the silencing of hypoxia-inducible Factor-1 alpha on metastasis of pancreatic cancer.Eur Rev Med Pharmacol Sci. 2013 Feb;17(4):436-46. Eur Rev Med Pharmacol Sci. 2013. PMID: 23467940
-
HIF-3α Promotes Metastatic Phenotypes in Pancreatic Cancer by Transcriptional Regulation of the RhoC-ROCK1 Signaling Pathway.Mol Cancer Res. 2018 Jan;16(1):124-134. doi: 10.1158/1541-7786.MCR-17-0256. Epub 2017 Sep 19. Mol Cancer Res. 2018. PMID: 28928287
-
Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer.Pharmacol Ther. 2016 Aug;164:152-69. doi: 10.1016/j.pharmthera.2016.04.009. Epub 2016 Apr 29. Pharmacol Ther. 2016. PMID: 27139518 Review.
Cited by
-
Salidroside ameliorated hypoxia-induced tumorigenesis of BxPC-3 cells via downregulating hypoxia-inducible factor (HIF)-1α and LOXL2.J Cell Biochem. 2020 Jan;121(1):165-173. doi: 10.1002/jcb.29000. Epub 2019 Jun 4. J Cell Biochem. 2020. PMID: 31162697 Free PMC article.
-
Restricted Water Diffusion in Diffusion-Weighted Magnetic Resonance Imaging in Pancreatic Cancer is Associated with Tumor Hypoxia.Cancers (Basel). 2020 Dec 30;13(1):89. doi: 10.3390/cancers13010089. Cancers (Basel). 2020. PMID: 33396818 Free PMC article.
-
Leucine drives LAT1-related SNAIL upregulation in glucose-starved pancreatic cancer cells.Med Mol Morphol. 2025 Mar;58(1):23-33. doi: 10.1007/s00795-024-00404-0. Epub 2024 Sep 6. Med Mol Morphol. 2025. PMID: 39240293
-
Car T Cells in Solid Tumors: Overcoming Obstacles.Int J Mol Sci. 2024 Apr 10;25(8):4170. doi: 10.3390/ijms25084170. Int J Mol Sci. 2024. PMID: 38673757 Free PMC article. Review.
-
Investigating the effects of stereotactic body radiation therapy on pancreatic tumor hypoxia and microvasculature in an orthotopic mouse model using intravital fluorescence microscopy.Sci Rep. 2024 Dec 28;14(1):31348. doi: 10.1038/s41598-024-82757-1. Sci Rep. 2024. PMID: 39733027 Free PMC article.
References
-
- Siegel R, Ward E, Brawley O, Jemal A. Cancer Statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. - PubMed
-
- Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362(17):1605–1617. - PubMed
-
- Pérez-Mancera PA, Guerra C, Barbacid M, Tuveson DA. What we have learned about pancreatic cancer from mouse models. Gastroenterology. 2012;142(5):1079–1092. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

