The macro domain as fusion tag for carrier-driven crystallization
- PMID: 27774698
- PMCID: PMC5275734
- DOI: 10.1002/pro.3073
The macro domain as fusion tag for carrier-driven crystallization
Abstract
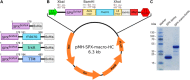
Obtaining well-ordered crystals remains a significant challenge in protein X-ray crystallography. Carrier-driven crystallization can facilitate crystal formation and structure solution of difficult target proteins. We obtained crystals of the small and highly flexible SPX domain from the yeast vacuolar transporter chaperone 4 (Vtc4) when fused to a C-terminal, non-cleavable macro tag derived from human histone macroH2A1.1. Initial crystals diffracted to 3.3 Å resolution. Reductive protein methylation of the fusion protein yielded a new crystal form diffracting to 2.1 Å. The structures were solved by molecular replacement, using isolated macro domain structures as search models. Our findings suggest that macro domain tags can be employed in recombinant protein expression in E. coli, and in carrier-driven crystallization.
Keywords: carrier-driven crystallization; crystallization tag; histone macroH2A; macro domain; recombinant protein expression.
© 2016 The Protein Society.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

