Emodin ameliorates bleomycin-induced pulmonary fibrosis in rats by suppressing epithelial-mesenchymal transition and fibroblast activation
- PMID: 27774992
- PMCID: PMC5075925
- DOI: 10.1038/srep35696
Emodin ameliorates bleomycin-induced pulmonary fibrosis in rats by suppressing epithelial-mesenchymal transition and fibroblast activation
Abstract
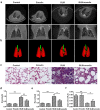
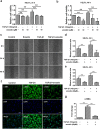
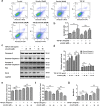
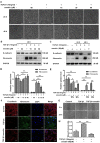
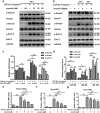
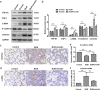
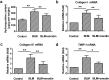
Aberrant activation of TGF-β1 is frequently encountered and promotes epithelial-mesenchymal transition (EMT) and fibroblast activation in pulmonary fibrosis. The present study investigated whether emodin mediates its effect via suppressing TGF-β1-induced EMT and fibroblast activation in bleomycin (BLM)-induced pulmonary fibrosis in rats. Here, we found that emodin induced apoptosis and inhibited cellular proliferation, migration and differentiation in TGF-β1-stimulated human embryonic lung fibroblasts (HELFs). Emodin suppressed TGF-β1-induced EMT in a dose- and time-dependent manner in alveolar epithelial A549 cells. Emodin also inhibited TGF-β1-induced Smad2, Smad3 and Erk1/2 activation, suggesting that Smad2/3 and Erk1/2 inactivation mediated the emodin-induced effects on TGF-β1-induced EMT. Additionally, we provided in vivo evidence suggesting that emodin apparently alleviated BLM-induced pulmonary fibrosis and improved pulmonary function by inhibiting TGF-β1 signaling and subsequently repressing EMT, fibroblast activation and extracellular matrix (ECM) deposition. Taken together, our data suggest that emodin mediates its effects mainly via inhibition of EMT and fibroblast activation and thus has a potential for the treatment of pulmonary fibrosis.
Figures
Similar articles
-
Ponatinib ameliorates pulmonary fibrosis by suppressing TGF-β1/Smad3 pathway.Pulm Pharmacol Ther. 2015 Oct;34:1-7. doi: 10.1016/j.pupt.2015.07.004. Epub 2015 Aug 5. Pulm Pharmacol Ther. 2015. PMID: 26254990
-
Emodin alleviates bleomycin-induced pulmonary fibrosis in rats.Toxicol Lett. 2016 Nov 16;262:161-172. doi: 10.1016/j.toxlet.2016.10.004. Epub 2016 Oct 4. Toxicol Lett. 2016. PMID: 27717887
-
Andrographolide ameliorates bleomycin-induced pulmonary fibrosis by suppressing cell proliferation and myofibroblast differentiation of fibroblasts via the TGF-β1-mediated Smad-dependent and -independent pathways.Toxicol Lett. 2020 Mar 15;321:103-113. doi: 10.1016/j.toxlet.2019.11.003. Epub 2019 Nov 6. Toxicol Lett. 2020. PMID: 31706003
-
[Research progress of anti-fibrotic drugs that inhibit epithelial-mesenchymal transition in pulmonary fibrosis].Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2023 Jan 20;41(1):72-77. doi: 10.3760/cma.j.cn121094-20210628-00308. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2023. PMID: 36725301 Review. Chinese.
-
ROS-Nrf2 pathway mediates the development of TGF-β1-induced epithelial-mesenchymal transition through the activation of Notch signaling.Eur J Cell Biol. 2021 Sep-Nov;100(7-8):151181. doi: 10.1016/j.ejcb.2021.151181. Epub 2021 Nov 3. Eur J Cell Biol. 2021. PMID: 34763128 Review.
Cited by
-
Utilizing MRI, [18F]FDG-PET and [89Zr]Zr-DFO-28H1 FAP-PET tracer to assess inflammation and fibrogenesis in a reproducible lung injury rat model: a multimodal imaging study.Front Nucl Med. 2023 Dec 12;3:1306251. doi: 10.3389/fnume.2023.1306251. eCollection 2023. Front Nucl Med. 2023. PMID: 39355041 Free PMC article.
-
TRPV4 regulates matrix stiffness and TGFβ1-induced epithelial-mesenchymal transition.J Cell Mol Med. 2019 Feb;23(2):761-774. doi: 10.1111/jcmm.13972. Epub 2018 Nov 18. J Cell Mol Med. 2019. PMID: 30450767 Free PMC article.
-
Total extract of Xin Jia Xuan Bai Cheng Qi decoction inhibits pulmonary fibrosis via the TGF-β/Smad signaling pathways in vivo and in vitro.Drug Des Devel Ther. 2019 Aug 19;13:2873-2886. doi: 10.2147/DDDT.S185418. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 31695321 Free PMC article.
-
Natural products as home-based prophylactic and symptom management agents in the setting of COVID-19.Phytother Res. 2020 Dec;34(12):3148-3167. doi: 10.1002/ptr.6794. Epub 2020 Aug 17. Phytother Res. 2020. PMID: 32881214 Free PMC article. Review.
-
Hydrogen sulfide attenuates cigarette smoke-induced airway remodeling by upregulating SIRT1 signaling pathway.Redox Biol. 2020 Jan;28:101356. doi: 10.1016/j.redox.2019.101356. Epub 2019 Oct 24. Redox Biol. 2020. PMID: 31704583 Free PMC article.
References
-
- King T. J. et al.. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 370, 2083–2092 (2014). - PubMed
-
- Xaubet A., Serrano-Mollar A. & Ancochea J. Pirfenidone for the treatment of idiopathic pulmonary fibrosis. Expert Opin Pharmacother. 15, 275–281 (2014). - PubMed
-
- Karimi-Shah B. A. & Chowdhury B. A. Forced vital capacity in idiopathic pulmonary fibrosis–FDA review of pirfenidone and nintedanib. N Engl J Med. 372, 1189–1191 (2015). - PubMed
-
- King T. J., Pardo A. & Selman M. Idiopathic pulmonary fibrosis. Lancet. 378, 1949–1961 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

