Extracellular vesicles from Paracoccidioides brasiliensis induced M1 polarization in vitro
- PMID: 27775058
- PMCID: PMC5075875
- DOI: 10.1038/srep35867
Extracellular vesicles from Paracoccidioides brasiliensis induced M1 polarization in vitro
Abstract
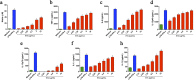
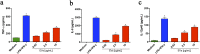
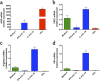
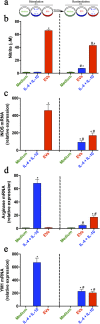
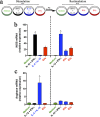
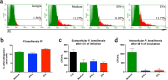
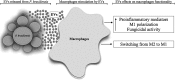
Extracellular vesicles (EVs) released by eukaryotes, archaea, and bacteria contain proteins, lipids, polysaccharides, and other molecules. The cargo analysis of EVs shows that they contain virulence factors suggesting a role in the pathogenesis of infection. The proteome, lipidome, RNA content, and carbohydrate composition of EVs from Paracoccidioides brasiliensis and Paracoccidioides lutzii were characterized. However, the effects of P. brasiliensis EVs on the host immune system have not yet been investigated. Herein, we verified that EVs from P. brasiliensis induce the production of proinflammatory mediators by murine macrophages in a dose-dependent manner. Addition of EV to macrophages also promoted transcription of the M1-polarization marker iNOs and diminish that of the M2 markers Arginase-1, Ym-1, and FIZZ-1. Furthermore, the augmented expression of M2-polarization markers, stimulated by IL-4 plus IL-10, was reverted toward an M1 phenotype in response to secondary stimulation with EVs from P. brasiliensis. The ability of EVs from P. brasiliensis to promote M1 polarization macrophages favoring an enhanced fungicidal activity, demonstrated by the decreased CFU recovery of internalized yeasts, with comparable phagocytic efficacy. Our results suggest that EVs from P. brasiliensis can modulate the innate immune response and affect the relationship between P. brasiliensis and host immune cells.
Figures
Similar articles
-
Protective Response in Experimental Paracoccidioidomycosis Elicited by Extracellular Vesicles Containing Antigens of Paracoccidioides brasiliensis.Cells. 2021 Jul 17;10(7):1813. doi: 10.3390/cells10071813. Cells. 2021. PMID: 34359982 Free PMC article.
-
Extracellular vesicles from virulent P. brasiliensis induce TLR4 and dectin-1 expression in innate cells and promote enhanced Th1/Th17 response.Virulence. 2024 Dec;15(1):2329573. doi: 10.1080/21505594.2024.2329573. Epub 2024 Mar 21. Virulence. 2024. PMID: 38511558 Free PMC article.
-
Extracellular Vesicles From the Dermatophyte Trichophyton interdigitale Modulate Macrophage and Keratinocyte Functions.Front Immunol. 2018 Oct 9;9:2343. doi: 10.3389/fimmu.2018.02343. eCollection 2018. Front Immunol. 2018. PMID: 30356863 Free PMC article.
-
Extracellular vesicles participate in macrophage-involved immune responses under liver diseases.Life Sci. 2020 Jan 1;240:117094. doi: 10.1016/j.lfs.2019.117094. Epub 2019 Nov 21. Life Sci. 2020. PMID: 31760101 Review.
-
Host- and Microbiota-Derived Extracellular Vesicles, Immune Function, and Disease Development.Int J Mol Sci. 2019 Dec 22;21(1):107. doi: 10.3390/ijms21010107. Int J Mol Sci. 2019. PMID: 31877909 Free PMC article. Review.
Cited by
-
Pathogenicity and Growth Conditions Modulate Fonsecaea Extracellular Vesicles' Ability to Interact With Macrophages.Front Cell Infect Microbiol. 2022 Jun 9;12:879018. doi: 10.3389/fcimb.2022.879018. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35755848 Free PMC article.
-
Fungal extracellular vesicle-mediated regulation: from virulence factor to clinical application.Front Microbiol. 2023 Sep 15;14:1205477. doi: 10.3389/fmicb.2023.1205477. eCollection 2023. Front Microbiol. 2023. PMID: 37779707 Free PMC article. Review.
-
Living Within the Macrophage: Dimorphic Fungal Pathogen Intracellular Metabolism.Front Cell Infect Microbiol. 2020 Oct 16;10:592259. doi: 10.3389/fcimb.2020.592259. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33178634 Free PMC article. Review.
-
Extracellular vesicles in phytopathogenic fungi.Extracell Vesicles Circ Nucl Acids. 2023 Mar 30;4(1):90-106. doi: 10.20517/evcna.2023.04. eCollection 2023. Extracell Vesicles Circ Nucl Acids. 2023. PMID: 39698296 Free PMC article. Review.
-
Protective effect of fungal extracellular vesicles against murine candidiasis.Cell Microbiol. 2020 Oct;22(10):e13238. doi: 10.1111/cmi.13238. Epub 2020 Jul 22. Cell Microbiol. 2020. PMID: 32558196 Free PMC article.
References
-
- Restrepo A., McEwen J. G. & Castaneda E. The habitat of Paracoccidioides brasiliensis: how far from solving the riddle? Med Mycol 39, 233–241 (2001). - PubMed
-
- Laniado-Laborin R. Coccidioidomycosis and other endemic mycoses in Mexico. Rev Iberoam Micol 24, 249–258, 200724249 [pii] (2007). - PubMed
-
- de Almeida S. M. Central nervous system paracoccidioidomycosis: an overview. Braz J Infect Dis 9, 126–133, S1413-86702005000200002 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

