CD133+ brain tumor-initiating cells are dependent on STAT3 signaling to drive medulloblastoma recurrence
- PMID: 27775079
- PMCID: PMC5541269
- DOI: 10.1038/onc.2016.235
CD133+ brain tumor-initiating cells are dependent on STAT3 signaling to drive medulloblastoma recurrence
Abstract
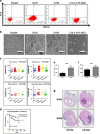
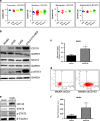
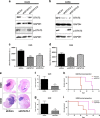
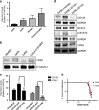
Medulloblastoma (MB), the most common malignant paediatric brain tumor, is currently treated using a combination of surgery, craniospinal radiotherapy and chemotherapy. Owing to MB stem cells (MBSCs), a subset of MB patients remains untreatable despite standard therapy. CD133 is used to identify MBSCs although its functional role in tumorigenesis has yet to be determined. In this work, we showed enrichment of CD133 in Group 3 MB is associated with increased rate of metastasis and poor clinical outcome. The signal transducers and activators of transcription-3 (STAT3) pathway are selectively activated in CD133+ MBSCs and promote tumorigenesis through regulation of c-MYC, a key genetic driver of Group 3 MB. We screened compound libraries for STAT3 inhibitors and treatment with the selected STAT3 inhibitors resulted in tumor size reduction in vivo. We propose that inhibition of STAT3 signaling in MBSCs may represent a potential therapeutic strategy to treat patients with recurrent MB.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Inhibition of phosphorylated STAT3 by cucurbitacin I enhances chemoradiosensitivity in medulloblastoma-derived cancer stem cells.Childs Nerv Syst. 2012 Mar;28(3):363-73. doi: 10.1007/s00381-011-1672-x. Epub 2012 Jan 17. Childs Nerv Syst. 2012. PMID: 22249380
-
Celecoxib suppresses the phosphorylation of STAT3 protein and can enhance the radiosensitivity of medulloblastoma-derived cancer stem-like cells.Int J Mol Sci. 2014 Jun 18;15(6):11013-29. doi: 10.3390/ijms150611013. Int J Mol Sci. 2014. PMID: 24945311 Free PMC article.
-
Effect of the STAT3 inhibitor STX-0119 on the proliferation of cancer stem-like cells derived from recurrent glioblastoma.Int J Oncol. 2013 Jul;43(1):219-27. doi: 10.3892/ijo.2013.1916. Epub 2013 Apr 23. Int J Oncol. 2013. PMID: 23612755
-
STAT3 as a Therapeutic Target for Glioblastoma.Anticancer Agents Med Chem. 2010 Sep;10(7):512-9. doi: 10.2174/187152010793498636. Anticancer Agents Med Chem. 2010. PMID: 20879983 Review.
-
Medulloblastoma stem cells: modeling tumor heterogeneity.Cancer Lett. 2013 Sep 10;338(1):23-31. doi: 10.1016/j.canlet.2012.07.010. Epub 2012 Jul 14. Cancer Lett. 2013. PMID: 22796365 Review.
Cited by
-
The natural polyphenol curcumin induces apoptosis by suppressing STAT3 signaling in esophageal squamous cell carcinoma.J Exp Clin Cancer Res. 2018 Dec 5;37(1):303. doi: 10.1186/s13046-018-0959-0. J Exp Clin Cancer Res. 2018. PMID: 30518397 Free PMC article.
-
Emerging impact of the long noncoding RNA MIR22HG on proliferation and apoptosis in multiple human cancers.J Exp Clin Cancer Res. 2020 Dec 3;39(1):271. doi: 10.1186/s13046-020-01784-8. J Exp Clin Cancer Res. 2020. PMID: 33267888 Free PMC article. Review.
-
The Curcumin Analogs 2-Pyridyl Cyclohexanone Induce Apoptosis via Inhibition of the JAK2-STAT3 Pathway in Human Esophageal Squamous Cell Carcinoma Cells.Front Pharmacol. 2018 Aug 21;9:820. doi: 10.3389/fphar.2018.00820. eCollection 2018. Front Pharmacol. 2018. PMID: 30186159 Free PMC article.
-
Marinopyrrole derivative MP1 as a novel anti-cancer agent in group 3 MYC-amplified Medulloblastoma.J Exp Clin Cancer Res. 2024 Jan 11;43(1):18. doi: 10.1186/s13046-024-02944-w. J Exp Clin Cancer Res. 2024. PMID: 38200580 Free PMC article.
-
PFKFB3 works on the FAK-STAT3-SOX2 axis to regulate the stemness in MPM.Br J Cancer. 2022 Oct;127(7):1352-1364. doi: 10.1038/s41416-022-01867-7. Epub 2022 Jul 6. Br J Cancer. 2022. PMID: 35794237 Free PMC article.
References
-
- Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol 2006; 7: 813–820. - PubMed
-
- Mabbott DJ, Spiegler BJ, Greenberg ML, Rutka JT, Hyder DJ, Bouffet E. Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. J Clin Oncol 2005; 23: 2256–2263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

