Incorporating Concomitant Medications into Genome-Wide Analyses for the Study of Complex Disease and Drug Response
- PMID: 27775101
- PMCID: PMC5013254
- DOI: 10.3389/fgene.2016.00138
Incorporating Concomitant Medications into Genome-Wide Analyses for the Study of Complex Disease and Drug Response
Abstract
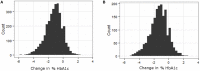
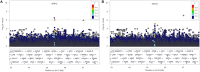
Given the high costs of conducting a drug-response trial, researchers are now aiming to use retrospective analyses to conduct genome-wide association studies (GWAS) to identify underlying genetic contributions to drug-response variation. To prevent confounding results from a GWAS to investigate drug response, it is necessary to account for concomitant medications, defined as any medication taken concurrently with the primary medication being investigated. We use data from the Action to Control Cardiovascular Disease (ACCORD) trial in order to implement a novel scoring procedure for incorporating concomitant medication information into a linear regression model in preparation for GWAS. In order to accomplish this, two primary medications were selected: thiazolidinediones and metformin because of the wide-spread use of these medications and large sample sizes available within the ACCORD trial. A third medication, fenofibrate, along with a known confounding medication, statin, were chosen as a proof-of-principle for the scoring procedure. Previous studies have identified SNP rs7412 as being associated with statin response. Here we hypothesize that including the score for statin as a covariate in the GWAS model will correct for confounding of statin and yield a change in association at rs7412. The response of the confounded signal was successfully diminished from p = 3.19 × 10-7 to p = 1.76 × 10-5, by accounting for statin using the scoring procedure presented here. This approach provides the ability for researchers to account for concomitant medications in complex trial designs where monotherapy treatment regimens are not available.
Keywords: bioinformatics; clinical trial; drug response; genomics; precision medicine.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

