The Molecular and Cellular Effect of Homocysteine Metabolism Imbalance on Human Health
- PMID: 27775595
- PMCID: PMC5085763
- DOI: 10.3390/ijms17101733
The Molecular and Cellular Effect of Homocysteine Metabolism Imbalance on Human Health
Abstract
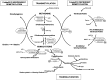
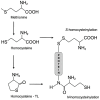
Homocysteine (Hcy) is a sulfur-containing non-proteinogenic amino acid derived in methionine metabolism. The increased level of Hcy in plasma, hyperhomocysteinemia, is considered to be an independent risk factor for cardio and cerebrovascular diseases. However, it is still not clear if Hcy is a marker or a causative agent of diseases. More and more research data suggest that Hcy is an important indicator for overall health status. This review represents the current understanding of molecular mechanism of Hcy metabolism and its link to hyperhomocysteinemia-related pathologies in humans. The aberrant Hcy metabolism could lead to the redox imbalance and oxidative stress resulting in elevated protein, nucleic acid and carbohydrate oxidation and lipoperoxidation, products known to be involved in cytotoxicity. Additionally, we examine the role of Hcy in thiolation of proteins, which results in their molecular and functional modifications. We also highlight the relationship between the imbalance in Hcy metabolism and pathogenesis of diseases, such as cardiovascular diseases, neurological and psychiatric disorders, chronic kidney disease, bone tissue damages, gastrointestinal disorders, cancer, and congenital defects.
Keywords: cellular toxicity; disease; homocysteine metabolism; hyperhomocysteimenia; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Stirzaker C., Song J.Z., Ng W., Du Q., Armstrong N.J., Locke W.J., Statham A.L., French H., Pidsley R., Valdes-Mora F., et al. Methyl-CpG-binding protein MBD2 plays a key role in maintenance and spread of DNA methylation at CpG islands and shores in cancer. Oncogen. 2016 doi: 10.1038/onc.2016.297. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

