Zinc Finger and X-Linked Factor (ZFX) Binds to Human SET Transcript 2 Promoter and Transactivates SET Expression
- PMID: 27775603
- PMCID: PMC5085766
- DOI: 10.3390/ijms17101737
Zinc Finger and X-Linked Factor (ZFX) Binds to Human SET Transcript 2 Promoter and Transactivates SET Expression
Abstract
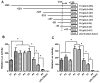
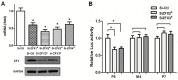
SET (SE Translocation) protein carries out multiple functions including those for protein phosphatase 2A (PP2A) inhibition, histone modification, DNA repair, and gene regulation. SET overexpression has been detected in brain neurons of patients suffering Alzheimer's disease, follicle theca cells of Polycystic Ovary Syndrome (PCOS) patients, and ovarian cancer cells, indicating that SET may play a pathological role for these disorders. SET transcript 2, produced by a specific promoter, represents a major transcript variant in different cell types. In this study, we characterized the transcriptional activation of human SET transcript 2 promoter in HeLa cells. Promoter deletion experiments and co-transfection assays indicated that ZFX, the Zinc finger and X-linked transcription factor, was able to transactivate the SET promoter. A proximal promoter region containing four ZFX-binding sites was found to be critical for the ZFX-mediated transactivation. Mutagenesis study indicated that the ZFX-binding site located the closest to the transcription start site accounted for most of the ZFX-mediated transactivity. Manipulation of ZFX levels by overexpression or siRNA knockdown confirmed the significance and specificity of the ZFX-mediated SET promoter activation. Chromatin immunoprecipitation results verified the binding of ZFX to its cognate sites in the SET promoter. These findings have led to identification of ZFX as an upstream factor regulating SET gene expression. More studies are required to define the in vivo significance of this mechanism, and specifically, its implication for several benign and malignant diseases related to SET dysregulation.
Keywords: I2PP2A (protein phosphatase 2A inhibitor); SET (SE Translocation); ZFX (Zinc finger and X-linked transcription factor); gynecologic cancers; transcriptional regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Von Lindern M., van Baal S., Wiegant J., Raap A., Hagemeijer A., Grosveld G. Can, a putative oncogene associated with myeloid leukemogenesis, may be activated by fusion of its 3′ half to different genes: Characterization of the set gene. Mol. Cell. Biol. 1992;12:3346–3355. doi: 10.1128/MCB.12.8.3346. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

