Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles
- PMID: 27775719
- PMCID: PMC5131849
- DOI: 10.1038/nn.4428
Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles
Erratum in
-
Corrigendum: Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles.Nat Neurosci. 2017 Jul 26;20(8):1189. doi: 10.1038/nn0817-1189a. Nat Neurosci. 2017. PMID: 28745723 No abstract available.
-
Publisher Correction: Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles.Nat Neurosci. 2020 Sep;23(9):1176. doi: 10.1038/s41593-020-0680-0. Nat Neurosci. 2020. PMID: 32661397
Abstract
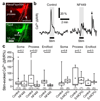
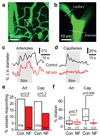
Active neurons increase their energy supply by dilating nearby arterioles and capillaries. This neurovascular coupling underlies blood oxygen level-dependent functional imaging signals, but its mechanism is controversial. Canonically, neurons release glutamate to activate metabotropic glutamate receptor 5 (mGluR5) on astrocytes, evoking Ca2+ release from internal stores, activating phospholipase A2 and generating vasodilatory arachidonic acid derivatives. However, adult astrocytes lack mGluR5, and knockout of the inositol 1,4,5-trisphosphate receptors that release Ca2+ from stores does not affect neurovascular coupling. We now show that buffering astrocyte Ca2+ inhibits neuronally evoked capillary dilation, that astrocyte [Ca2+]i is raised not by release from stores but by entry through ATP-gated channels, and that Ca2+ generates arachidonic acid via phospholipase D2 and diacylglycerol lipase rather than phospholipase A2. In contrast, dilation of arterioles depends on NMDA receptor activation and Ca2+-dependent NO generation by interneurons. These results reveal that different signaling cascades regulate cerebral blood flow at the capillary and arteriole levels.
Conflict of interest statement
Competing Financial Interests: The authors declare no competing interests.
Figures





Comment in
-
Astrocyte endfeet march to the beat of different vessels.Nat Neurosci. 2016 Nov 29;19(12):1539-1541. doi: 10.1038/nn.4446. Nat Neurosci. 2016. PMID: 27898083 No abstract available.
References
-
- Zonta M, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. - PubMed
-
- Mulligan SJ, Macvicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. - PubMed
-
- Takano T, et al. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. - PubMed
-
- Zimmermann KW. Der Feinere Bau der Blutcapillaren. Zeitschrift fur Anatomie und Entwicklungsgeschichte. 1923;68:29–109.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

