Genome-wide significance testing of variation from single case exomes
- PMID: 27776118
- PMCID: PMC5127779
- DOI: 10.1038/ng.3697
Genome-wide significance testing of variation from single case exomes
Abstract
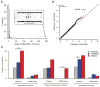
Standard techniques from genetic epidemiology are ill-suited to formally assess the significance of variants identified from a single case. We developed a statistical inference framework for identifying unusual functional variation from a single exome or genome, what we refer to as the 'n-of-one' problem. Using this approach we assessed our ability to identify the causal genotypes in over 5 million simulated cases of Mendelian disease, identifying 39% of disease genotypes as the most damaging unit in a typical exome background. We applied our approach to 129 n-of-one families from the Undiagnosed Diseases Program, nominating 60% of 30 disease genes determined to be diagnostic by a standard clinical workup. Our method can currently produce well-calibrated P values when applied to single genomes, can facilitate integration of multiple data types for n-of-one analyses, and, with further work, could become a widely used epidemiological method like linkage analysis or genome-wide association analysis.
Conflict of interest statement
D.F.C is funded by a research contract with PierianDx to develop novel methods for clinical exome analysis.
Figures


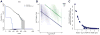

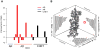
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

