Tyrosine Binding Protein Sites Regulate the Intracellular Trafficking and Processing of Amyloid Precursor Protein through a Novel Lysosome-Directed Pathway
- PMID: 27776132
- PMCID: PMC5077117
- DOI: 10.1371/journal.pone.0161445
Tyrosine Binding Protein Sites Regulate the Intracellular Trafficking and Processing of Amyloid Precursor Protein through a Novel Lysosome-Directed Pathway
Abstract
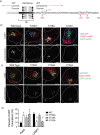
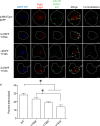
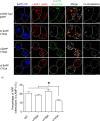
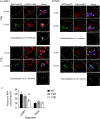
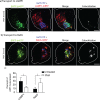
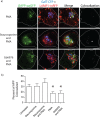
The amyloid hypothesis posits that the production of β-amyloid (Aβ) aggregates leads to neurodegeneration and cognitive decline associated with AD. Aβ is produced by sequential cleavage of the amyloid precursor protein (APP) by β- and γ-secretase. While nascent APP is well known to transit to the endosomal/ lysosomal system via the cell surface, we have recently shown that APP can also traffic to lysosomes intracellularly via its interaction with AP-3. Because AP-3 interacts with cargo protein via interaction with tyrosine motifs, we mutated the three tyrosines motif in the cytoplasmic tail of APP. Here, we show that the YTSI motif interacts with AP-3, and phosphorylation of the serine in this motif disrupts the interaction and decreases APP trafficking to lysosomes. Furthermore, we show that phosphorylation at this motif can decrease the production of neurotoxic Aβ 42. This demonstrates that reducing APP trafficking to lysosomes may be a strategy to reduce Aβ 42 in Alzheimer's disease.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Petanceska SS, Seeger M, Checler F, Gandy S. Mutant presenilin 1 increases the levels of Alzheimer amyloid beta-peptide Abeta42 in late compartments of the constitutive secretory pathway. Journal of Neurochemistry. 2000;74: 1878–1884. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

