Systematic Review with Network Meta-Analysis: Comparative Efficacy of Biologics in the Treatment of Moderately to Severely Active Ulcerative Colitis
- PMID: 27776175
- PMCID: PMC5077077
- DOI: 10.1371/journal.pone.0165435
Systematic Review with Network Meta-Analysis: Comparative Efficacy of Biologics in the Treatment of Moderately to Severely Active Ulcerative Colitis
Abstract
Background: Biological therapies are increasingly used to treat ulcerative colitis (UC).
Aim: To compare the efficacy of biologics in adults with moderately-to-severely active UC, stratified by prior exposure to anti-tumour necrosis factor (anti-TNF) therapy.
Methods: A systematic literature review was undertaken to identify studies of biologics approved for UC. Network meta-analysis was conducted for endpoints at induction and maintenance.
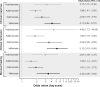
Results: Seven studies were included in the meta-analysis of induction treatment for anti-TNF therapy-naïve patients. All biologics were more effective than placebo in inducing clinical response, clinical remission, and mucosal healing. Infliximab demonstrated a statistically significant improvement over adalimumab in clinical response (odds ratio [OR] [95% credible interval (CrI)]: 2.19 [1.35-3.55]), clinical remission (OR [95% CrI]: 2.81 [1.49-5.49]), and mucosal healing (OR [95% CrI]: 2.23 [1.21-4.14]); there were no other significant differences between biologics for induction efficacy. Five studies were included in the meta-analysis of maintenance treatment, two studies rerandomised responder patients at end of induction, and three followed the same patients 'straight through'. To account for design differences, the number of responders at end of induction was assumed to be equivalent to the number rerandomised. Vedolizumab showed significantly different durable clinical response from comparators (OR [95% CrI] infliximab 3.18 [1.14-9.20], golimumab 2.33 [1.04-5.41], and adalimumab 3.96 [1.67-9.84]). In anti-TNF therapy-experienced patients, only vedolizumab and adalimumab could be compared. At induction, no significant differences in efficacy were seen. During maintenance, vedolizumab showed significantly improved rates of mucosal healing versus adalimumab (OR [95% CrI]: 6.72 [1.36-41.0]).
Conclusions: This study expands the understanding of comparative efficacies of biologic treatments for UC, encompassing outcomes and populations not previously studied. All biologic treatments were effective for UC during induction. Vedolizumab demonstrated possible clinical benefits in the maintenance setting versus all comparators, irrespective of prior anti-TNF exposure and after adjusting for differences in study design.
Conflict of interest statement
Claire Ainsworth, Adrian D. Vickers and Caroline S. Ling are employees of RTI Health Solutions. Annika Bergmann, Jasmina Medjedovic and Michael Smyth are employees of Takeda Pharmaceuticals. Reema Mody was an employee of Takeda Pharmaceuticals during the conduct of the study. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures





References
-
- Crohn’s and Colitis UK. Ulcerative colitis Ed 8a—November 2013. Available: https://www.crohnsandcolitis.org.uk/about-inflammatory-bowel-disease/pub.... Accessed 26 May 2016.
-
- National Institutes of Health. Ulcerative colitis. June 2010. Available: http://www.nlm.nih.gov/medlineplus/ulcerativecolitis.html. Accessed 4 Sep 2012.
-
- National Institute for Health and Care Excellence. Ulcerative colitis: management in adults, children and young people. June 2013. Available: https://www.nice.org.uk/guidance/cg166/chapter/introduction. Accessed 9 Apr 2015.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

