Olfactory receptor pseudo-pseudogenes
- PMID: 27776356
- PMCID: PMC5164928
- DOI: 10.1038/nature19824
Olfactory receptor pseudo-pseudogenes
Abstract
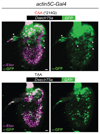
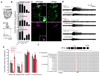
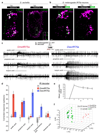
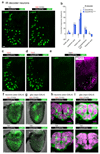
Pseudogenes are generally considered to be non-functional DNA sequences that arise through nonsense or frame-shift mutations of protein-coding genes. Although certain pseudogene-derived RNAs have regulatory roles, and some pseudogene fragments are translated, no clear functions for pseudogene-derived proteins are known. Olfactory receptor families contain many pseudogenes, which reflect low selection pressures on loci no longer relevant to the fitness of a species. Here we report the characterization of a pseudogene in the chemosensory variant ionotropic glutamate receptor repertoire of Drosophila sechellia, an insect endemic to the Seychelles that feeds almost exclusively on the ripe fruit of Morinda citrifolia. This locus, D. sechellia Ir75a, bears a premature termination codon (PTC) that appears to be fixed in the population. However, D. sechellia Ir75a encodes a functional receptor, owing to efficient translational read-through of the PTC. Read-through is detected only in neurons and is independent of the type of termination codon, but depends on the sequence downstream of the PTC. Furthermore, although the intact Drosophila melanogaster Ir75a orthologue detects acetic acid-a chemical cue important for locating fermenting food found only at trace levels in Morinda fruit-D. sechellia Ir75a has evolved distinct odour-tuning properties through amino-acid changes in its ligand-binding domain. We identify functional PTC-containing loci within different olfactory receptor repertoires and species, suggesting that such 'pseudo-pseudogenes' could represent a widespread phenomenon.
Conflict of interest statement
Declared none.
Figures


Comment in
-
Evolutionary Genetics: Smells like a Pseudo-pseudogene.Curr Biol. 2016 Dec 19;26(24):R1294-R1296. doi: 10.1016/j.cub.2016.11.006. Curr Biol. 2016. PMID: 27997845
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

