Respiratory microbes present in the nasopharynx of children hospitalised with suspected pulmonary tuberculosis in Cape Town, South Africa
- PMID: 27776489
- PMCID: PMC5075757
- DOI: 10.1186/s12879-016-1934-z
Respiratory microbes present in the nasopharynx of children hospitalised with suspected pulmonary tuberculosis in Cape Town, South Africa
Abstract
Background: Lower respiratory tract infection in children is increasingly thought to be polymicrobial in origin. Children with symptoms suggestive of pulmonary tuberculosis (PTB) may have tuberculosis, other respiratory tract infections or co-infection with Mycobacterium tuberculosis and other pathogens. We aimed to identify the presence of potential respiratory pathogens in nasopharyngeal (NP) samples from children with suspected PTB.
Method: NP samples collected from consecutive children presenting with suspected PTB at Red Cross Children's Hospital (Cape Town, South Africa) were tested by multiplex real-time RT-PCR. Mycobacterial liquid culture and Xpert MTB/RIF was performed on 2 induced sputa obtained from each participant. Children were categorised as definite-TB (culture or qPCR [Xpert MTB/RIF] confirmed), unlikely-TB (improvement of symptoms without TB treatment on follow-up) and unconfirmed-TB (all other children).
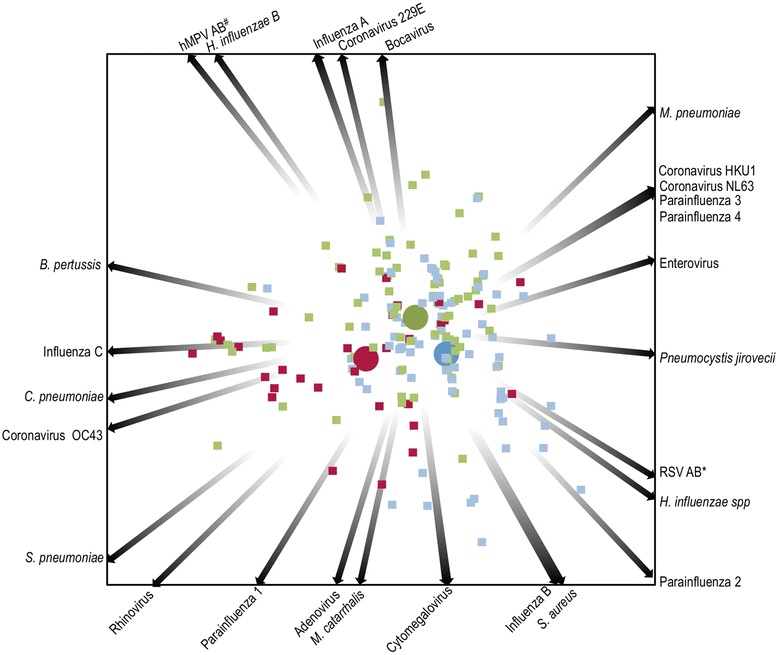
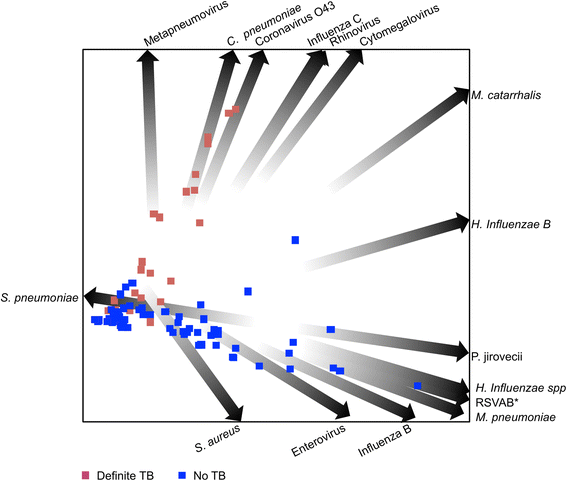
Results: Amongst 214 children with a median age of 36 months (interquartile range, [IQR] 19-66 months), 34 (16 %) had definite-TB, 86 (40 %) had unconfirmed-TB and 94 (44 %) were classified as unlikely-TB. Moraxella catarrhalis (64 %), Streptococcus pneumoniae (42 %), Haemophilus influenzae spp (29 %) and Staphylococcus aureus (22 %) were the most common bacteria detected in NP samples. Other bacteria detected included Mycoplasma pneumoniae (9 %), Bordetella pertussis (7 %) and Chlamydophila pneumoniae (4 %). The most common viruses detected included metapneumovirus (19 %), rhinovirus (15 %), influenza virus C (9 %), adenovirus (7 %), cytomegalovirus (7 %) and coronavirus O43 (5.6 %). Both bacteria and viruses were detected in 73, 55 and 56 % of the definite, unconfirmed and unlikely-TB groups, respectively. There were no significant differences in the distribution of respiratory microbes between children with and without TB. Using quadratic discriminant analysis, human metapneumovirus, C. pneumoniae, coronavirus 043, influenza virus C virus, rhinovirus and cytomegalovirus best discriminated children with definite-TB from the other groups of children.
Conclusions: A broad range of potential respiratory pathogens was detected in children with suspected TB. There was no clear association between TB categorisation and detection of a specific pathogen. Further work is needed to explore potential pathogen interactions and their role in the pathogenesis of PTB.
Keywords: Infection; Microbiota; Mycobacterium tuberculosis; Nasopharynx; Respiratory microbes; Tuberculosis.
Figures
References
-
- Zar HJ, Barnett W, Stadler A, Gardner-Lubbe S, Myer L, et al. Aetiology of childhood pneumonia in a well vaccinated South African birth cohort: a nested case-control study of the Drakenstein Child Health Study. Lancet Respir Med. 2016;4:463–72. doi: 10.1016/S2213-2600(16)00096-5. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

