Moxibustion treatment for diarrhea-predominant irritable bowel syndrome: study protocol for a randomized controlled trial
- PMID: 27776494
- PMCID: PMC5075981
- DOI: 10.1186/s12906-016-1386-4
Moxibustion treatment for diarrhea-predominant irritable bowel syndrome: study protocol for a randomized controlled trial
Abstract
Background: Irritable bowel syndrome (IBS) is a worldwide disease with high morbidity. The effect of current treatment with Western medicine is not satisfactory. Although moxibustion treatment is widely used for gastrointestinal diseases, randomized controlled trials on the use of this treatment for IBS are limited. This study aims to evaluate the clinical efficacy and safety of moxibustion treatment in patients with diarrhea-predominant irritable bowel syndrome (IBS-D).
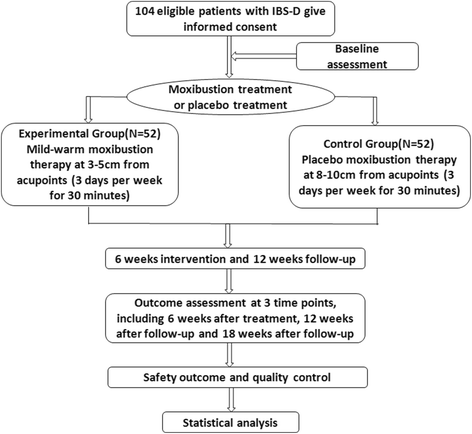
Methods/design: A multi-center, randomized, single-blind and placebo-controlled trial is employed. 104 cases will be divided into two groups: (1) a mild-warm moxibustion group in which moxa stick is 3-5 cm away from acupuncture points and the skin temperature is maintained at 43 ± 1 °C; and (2) a placebo moxibustion group in which moxa stick is 8-10 cm away from acupuncture points and the skin temperature is maintained at 37 ± 1 °C. Moxibustion is performed on bilateral ST25 and ST36 in the two groups for 30 min each time, three times a week for 6 weeks. The patients are followed up at the 12th and 18th weeks. Adequate relief is used as a primary outcome measure; IBS symptom severity score, Bristol stool form scale, IBS quality-of-life questionnaire, and hospital anxiety and depression scale are used as secondary outcome measures.
Discussion: This study aims to demonstrate the safety and efficacy of moxibustion treatment for IBS-D, which may validate moxibustion as an effective therapy for treating IBS-D.
Trial registration: ClinicalTrials.gov identifier NCT02421627 (8 April 2015).
Keywords: Diarrhea; Irritable bowel syndrome; Moxibustion; Randomized controlled trial.
Similar articles
-
Mild moxibustion for Irritable Bowel Syndrome with Diarrhea (IBS-D): A randomized controlled trial.J Ethnopharmacol. 2022 May 10;289:115064. doi: 10.1016/j.jep.2022.115064. Epub 2022 Jan 31. J Ethnopharmacol. 2022. PMID: 35114338 Clinical Trial.
-
[Herbal-moxa plaster for diarrhea type irritable bowel syndrome of spleen and kidney yang deficiency: a randomized controlled trial].Zhongguo Zhen Jiu. 2023 Jun 12;43(6):617-21. doi: 10.13703/j.0255-2930.20220804-k0001. Zhongguo Zhen Jiu. 2023. PMID: 37313553 Clinical Trial. Chinese.
-
Long-term effect of moxibustion on irritable bowel syndrome with diarrhea: a randomized clinical trial.Therap Adv Gastroenterol. 2022 Feb 23;15:17562848221075131. doi: 10.1177/17562848221075131. eCollection 2022. Therap Adv Gastroenterol. 2022. PMID: 35222693 Free PMC article.
-
Moxibustion for diarrhea-predominant irritable bowel syndrome: A systematic review and meta-analysis of randomized controlled trials.Complement Ther Clin Pract. 2022 Feb;46:101532. doi: 10.1016/j.ctcp.2021.101532. Epub 2022 Jan 3. Complement Ther Clin Pract. 2022. PMID: 35051805
-
Update on the Management of Diarrhea-Predominant Irritable Bowel Syndrome: Focus on Rifaximin and Eluxadoline.Pharmacotherapy. 2016 Mar;36(3):300-16. doi: 10.1002/phar.1712. Epub 2016 Mar 11. Pharmacotherapy. 2016. PMID: 26971716 Review.
Cited by
-
Clinical evidence of acupuncture and moxibustion for irritable bowel syndrome: A systematic review and meta-analysis of randomized controlled trials.Front Public Health. 2022 Nov 24;10:1022145. doi: 10.3389/fpubh.2022.1022145. eCollection 2022. Front Public Health. 2022. PMID: 36589968 Free PMC article.
-
Research Trends of Moxibustion Therapy for Pain Treatment Over the Past Decade: A Bibliometric Analysis.J Pain Res. 2022 Aug 20;15:2465-2479. doi: 10.2147/JPR.S374564. eCollection 2022. J Pain Res. 2022. PMID: 36035980 Free PMC article. Review.
-
Efficacy and safety of Chinese botanical drug Si Shen Wan in irritable bowel syndrome: a meta-analysis and trial sequential analysis of randomized controlled trials.Front Pharmacol. 2025 Jun 2;16:1534904. doi: 10.3389/fphar.2025.1534904. eCollection 2025. Front Pharmacol. 2025. PMID: 40529486 Free PMC article.
-
Vitamin D supplementation in adolescents with irritable bowel syndrome: Is it useful? A randomized controlled trial.Saudi J Gastroenterol. 2018 Mar-Apr;24(2):109-114. doi: 10.4103/sjg.SJG_438_17. Saudi J Gastroenterol. 2018. PMID: 29637918 Free PMC article. Clinical Trial.
-
Vitamin D improves irritable bowel syndrome symptoms: A meta-analysis.Heliyon. 2023 May 25;9(6):e16437. doi: 10.1016/j.heliyon.2023.e16437. eCollection 2023 Jun. Heliyon. 2023. PMID: 37260904 Free PMC article.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous