Biofilm as a production platform for heterologous production of rhamnolipids by the non-pathogenic strain Pseudomonas putida KT2440
- PMID: 27776509
- PMCID: PMC5075983
- DOI: 10.1186/s12934-016-0581-9
Biofilm as a production platform for heterologous production of rhamnolipids by the non-pathogenic strain Pseudomonas putida KT2440
Abstract
Background: Although a transition toward sustainable production of chemicals is needed, the physiochemical properties of certain biochemicals such as biosurfactants make them challenging to produce in conventional bioreactor systems. Alternative production platforms such as surface-attached biofilm populations could potentially overcome these challenges. Rhamnolipids are a group of biosurfactants highly relevant for industrial applications. However, they are mainly produced by the opportunistic pathogen Pseudomonas aeruginosa using hydrophobic substrates such as plant oils. As the biosynthesis is tightly regulated in P. aeruginosa a heterologous production of rhamnolipids in a safe organism can relive the production from many of these limitations and alternative substrates could be used.
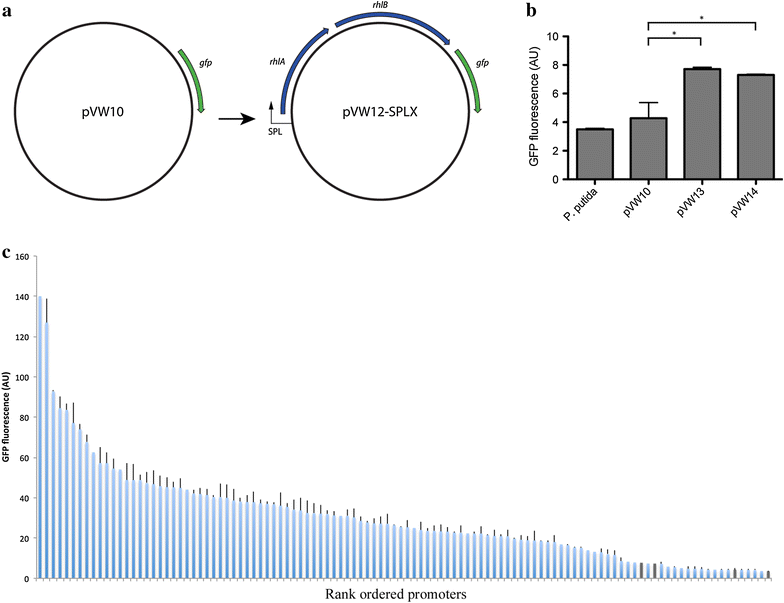
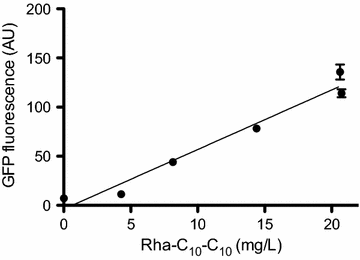
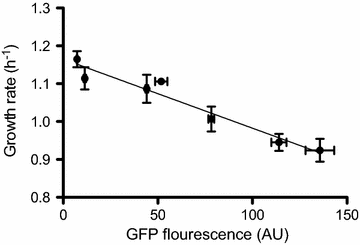
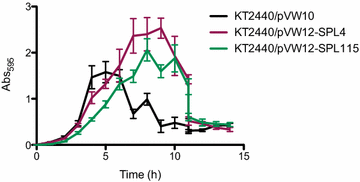
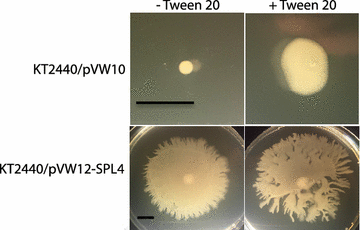
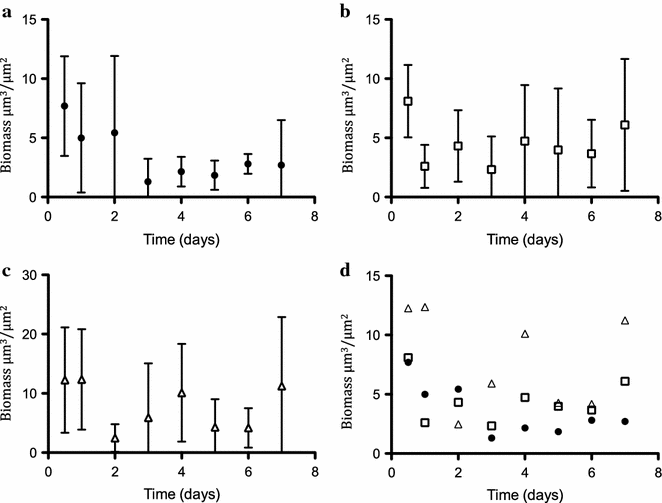
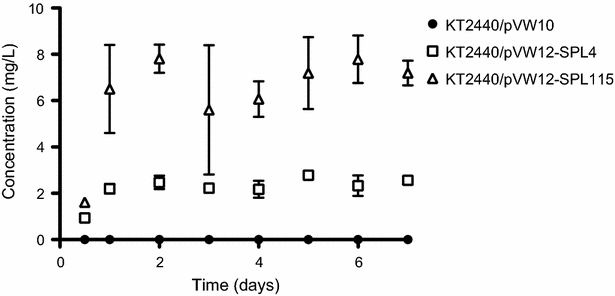
Results: In the present study, heterologous production of biosurfactants was investigated using rhamnolipids as the model compound in biofilm encased Pseudomonas putida KT2440. The rhlAB operon from P. aeruginosa was introduced into P. putida to produce mono-rhamnolipids. A synthetic promoter library was used in order to bypass the normal regulation of rhamnolipid synthesis and to provide varying expression levels of the rhlAB operon resulting in different levels of rhamnolipid production. Biosynthesis of rhamnolipids in P. putida decreased bacterial growth rate but stimulated biofilm formation by enhancing cell motility. Continuous rhamnolipid production in a biofilm was achieved using flow cell technology. Quantitative and structural investigations of the produced rhamnolipids were made by ultra performance liquid chromatography combined with high resolution mass spectrometry (HRMS) and tandem HRMS. The predominant rhamnolipid congener produced by the heterologous P. putida biofilm was mono-rhamnolipid with two C10 fatty acids.
Conclusion: This study shows a successful application of synthetic promoter library in P. putida KT2440 and a heterologous biosynthesis of rhamnolipids in biofilm encased cells without hampering biofilm capabilities. These findings expands the possibilities of cultivation setups and paves the way for employing biofilm flow systems as production platforms for biochemicals, which as a consequence of physiochemical properties are troublesome to produce in conventional fermenter setups, or for production of compounds which are inhibitory or toxic to the production organisms.
Keywords: Biofilm; Biosurfactants; Heterologous biosynthesis; Pseudomonas putida KT2440; Rhamnolipids; Synthetic promoter library; UHPLC-HRMS; UHPLC-MS/HRMS.
Figures
References
-
- Henkel M, Müller MM, Kügler JH, Lovaglio RB, Contiero J, Syldatk C, Hausmann R. Rhamnolipids as biosurfactants from renewable resources: concepts for next-generation rhamnolipid production. Process Biochem. 2012;47:1207–1219. doi: 10.1016/j.procbio.2012.04.018. - DOI
-
- Jarvis F, Johnson M. A glyco-lipide produced by Pseudomonas aeruginosa. J Am Chem Soc. 1949;71:4124–4126. doi: 10.1021/ja01180a073. - DOI
-
- Deziel E, Lepine F, Dennie D, Boismenu D, Mamer OA, Villemur R. Liquid chromatography/mass spectrometry analysis of mixtures of rhamnolipids produced by Pseudomonas aeruginosa strain 57RP grown on mannitol or naphthalene. Biochim Biophys Acta. 1999;1440:244–252. doi: 10.1016/S1388-1981(99)00129-8. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

