Neurological manifestations of autosomal dominant familial Alzheimer's disease: a comparison of the published literature with the Dominantly Inherited Alzheimer Network observational study (DIAN-OBS)
- PMID: 27777020
- PMCID: PMC5116769
- DOI: 10.1016/S1474-4422(16)30229-0
Neurological manifestations of autosomal dominant familial Alzheimer's disease: a comparison of the published literature with the Dominantly Inherited Alzheimer Network observational study (DIAN-OBS)
Erratum in
-
Corrections.Lancet Neurol. 2017 Jan;16(1):24. doi: 10.1016/S1474-4422(16)30327-1. Epub 2016 Nov 16. Lancet Neurol. 2017. PMID: 27864006 No abstract available.
Abstract
Background: Autosomal dominant familial Alzheimer's disease (ADAD) is a rare disorder with non-amnestic neurological symptoms in some clinical presentations. We aimed to compile and compare data from symptomatic participants in the Dominantly Inherited Alzheimer Network observational study (DIAN-OBS) with those reported in the literature to estimate the prevalences of non-amnestic neurological symptoms in participants with ADAD.
Methods: We prospectively collected data from the DIAN-OBS database, which recruited participants from study centres in the USA, Europe, and Australia, between Feb 29, 2008, and July 1, 2014. We also did a systematic review of publications to extract individual-level clinical data for symptomatic participants with ADAD. We used data for age of onset (from first report of cognitive decline), disease course from onset to death, and the presence of 13 neurological findings that have been reported in association with ADAD. Using multivariable linear regression, we investigated the prevalences of various non-amnestic neurological symptoms and the contributions of age of onset and specific mutation type on symptoms.
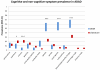
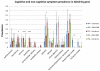
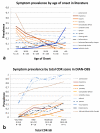
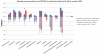
Findings: The DIAN-OBS dataset included 107 individuals with detailed clinical data (forming the DIAN-OBS cohort). Our systematic review yielded 188 publications reporting on 1228 symptomatic individuals, with detailed neurological examination descriptions available for 753 individuals (forming the published data cohort). The most prevalent non-amnestic cognitive manifestations in participants in the DIAN-OBS cohort were those typical of mild to moderate Alzheimer's disease, including visual agnosia (55·1%, 95% CI 45·7-64·6), aphasia (57·9%, 48·6-67·3), and behavioural changes (61·7%, 51·5-70·0). Non-amnestic cognitive manifestations were less prevalent in the published data cohort (eg, visual agnosia [5·6%, 3·9-7·2], aphasia [23·0%, 20·0-26·0], and behavioural changes [31·7%, 28·4-35·1]). Prevalence of non-cognitive neurological manifestations in the DIAN-OBS cohort was low, including myoclonus and spasticity (9·3%, 95% CI 3·8-15·0), and seizures (2·8%, 0·5-5·9) and moderate for parkinsonism (11·2%, 5·3-17·1). By constrast, prevalence was higher in the published data cohort for myoclonus and spasticity (19·4%, 16·6-22·2 and 15·0%, 12·5-17·6, respectively), parkinsonism (12·5%, 10·1-15·0), and seizures (20·3%, 17·4-23·2). In an analysis of the published data cohort, ischaemic stroke was more prevalent at older ages of onset of symptoms of ADAD (odds ratio 1·09 per 1 year increase in age of onset, 95% CI 1·04-1·14, p=0·0003); and motor symptoms were more common at younger age of onset (myoclonus 0·93, 0·90-0·97, p=0·0007; seizures 0·95, 0·92-0·98, p=0·0018; corticobulbar deficits 0·91, 0·86-0·96, p=0·0012; and cerebellar ataxia 0·82, 0·74-0·91, p=0·0002). In the DIAN-OBS cohort, non-cognitive symptoms were more common at more severe stages of disease.
Interpretation: The non-cognitive clinical manifestations of Alzheimer's disease seem to affect a small proportion of participants with mild to moderate ADAD, and are probably influenced by disease severity, environmental, and genetic factors. When evaluating patients with potential ADAD, clinicians should note that cognitive symptoms typical of sporadic Alzheimer's disease are the most consistent finding, with some patients manifesting non-cognitive neurological symptoms. Future work is needed to determine the environmental and genetic factors that cause these neurological symptoms.
Funding: National Institutes of Health and German Center for Neurodegenerative Diseases.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures




Comment in
-
Clinical heterogeneity in familial Alzheimer's disease.Lancet Neurol. 2016 Dec;15(13):1296-1298. doi: 10.1016/S1474-4422(16)30275-7. Epub 2016 Oct 21. Lancet Neurol. 2016. PMID: 27777021 No abstract available.
References
-
- Larner AJ, Doran M. Clinical phenotypic heterogeneity of Alzheimer’s disease associated with mutations of the presenilin-1 gene. J Neurol. 2006;253:139–58. - PubMed
-
- Larner AJ, Doran M. Genotype-phenotype relationships of presenilin-1 mutations in Alzheimer’s disease: an update. J Alzheimers Dis. 2009;17:259–65. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

