Individualized therapy for persistent asthma in young children
- PMID: 27777180
- PMCID: PMC5148729
- DOI: 10.1016/j.jaci.2016.09.028
Individualized therapy for persistent asthma in young children
Abstract
Background: Phenotypic presentations in young children with asthma are varied and might contribute to differential responses to asthma controller medications.
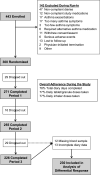
Methods: The Individualized Therapy for Asthma in Toddlers study was a multicenter, randomized, double-blind, double-dummy clinical trial in children aged 12 to 59 months (n = 300) with asthma necessitating treatment with daily controller (Step 2) therapy. Participants completed a 2- to 8-week run-in period followed by 3 crossover periods with daily inhaled corticosteroids (ICSs), daily leukotriene receptor antagonists, and as-needed ICS treatment coadministered with albuterol. The primary outcome was differential response to asthma medication based on a composite measure of asthma control. The primary analysis involved 2 stages: determination of differential response and assessment of whether 3 prespecified features (aeroallergen sensitization, previous exacerbations, and sex) predicted a differential response.
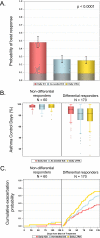
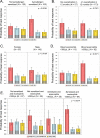
Results: Seventy-four percent (170/230) of children with analyzable data had a differential response to the 3 treatment strategies. Within differential responders, the probability of best response was highest for a daily ICS and was predicted by aeroallergen sensitization but not exacerbation history or sex. The probability of best response to daily ICS was further increased in children with both aeroallergen sensitization and blood eosinophil counts of 300/μL or greater. In these children daily ICS use was associated with more asthma control days and fewer exacerbations compared with the other treatments.
Conclusions: In young children with asthma necessitating Step 2 treatment, phenotyping with aeroallergen sensitization and blood eosinophil counts is useful for guiding treatment selection and identifies children with a high exacerbation probability for whom treatment with a daily ICS is beneficial despite possible risks of growth suppression.
Trial registration: ClinicalTrials.gov NCT01606306.
Keywords: Asthma; asthma biomarkers; asthma phenotype; asthma treatment; inhaled corticosteroid; leukotriene receptor antagonist; personalized medicine; treatment response.
Copyright © 2016 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures


Comment in
-
Nonatopic persistent asthma in children, a missed phenotype of asthma?J Allergy Clin Immunol. 2017 Oct;140(4):1212-1213. doi: 10.1016/j.jaci.2017.06.019. Epub 2017 Aug 3. J Allergy Clin Immunol. 2017. PMID: 28780971 No abstract available.
-
Reply.J Allergy Clin Immunol. 2017 Oct;140(4):1213. doi: 10.1016/j.jaci.2017.06.018. Epub 2017 Aug 3. J Allergy Clin Immunol. 2017. PMID: 28780972 No abstract available.
References
-
- [March 14, 2016];From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2015 Available: http://www.ginasthma.org/.
-
- National Asthma Education and Prevention Panel Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. The Journal of allergy and clinical immunology. 2007;120:S94–138. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 HL098107/HL/NHLBI NIH HHS/United States
- UG1 HD090878/HD/NICHD NIH HHS/United States
- U10 HL098075/HL/NHLBI NIH HHS/United States
- K23 AI104780/AI/NIAID NIH HHS/United States
- K23 AI106945/AI/NIAID NIH HHS/United States
- U10 HL098102/HL/NHLBI NIH HHS/United States
- U10 HL098115/HL/NHLBI NIH HHS/United States
- U10 HL098103/HL/NHLBI NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- U10 HL098090/HL/NHLBI NIH HHS/United States
- U10 HL098098/HL/NHLBI NIH HHS/United States
- U10 HL064313/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

