Evaluation of computed tomography in patients with atypical angina or chest pain clinically referred for invasive coronary angiography: randomised controlled trial
- PMID: 27777234
- PMCID: PMC5076567
- DOI: 10.1136/bmj.i5441
Evaluation of computed tomography in patients with atypical angina or chest pain clinically referred for invasive coronary angiography: randomised controlled trial
Erratum in
-
Evaluation of computed tomography in patients with atypical angina or chest pain clinically referred for invasive coronary angiography: randomised controlled trial.BMJ. 2016 Nov 29;355:i6420. doi: 10.1136/bmj.i6420. BMJ. 2016. PMID: 27899352 Free PMC article. No abstract available.
Abstract
Objective: To evaluate whether invasive coronary angiography or computed tomography (CT) should be performed in patients clinically referred for coronary angiography with an intermediate probability of coronary artery disease.
Design: Prospective randomised single centre trial.
Setting: University hospital in Germany.
Participants: 340 patients with suspected coronary artery disease and a clinical indication for coronary angiography on the basis of atypical angina or chest pain.
Interventions: 168 patients were randomised to CT and 172 to coronary angiography. After randomisation one patient declined CT and 10 patients declined coronary angiography, leaving 167 patients (88 women) and 162 patients (78 women) for analysis. Allocation could not be blinded, but blinded independent investigators assessed outcomes.
Main outcome measure: The primary outcome measure was major procedural complications within 48 hours of the last procedure related to CT or angiography.
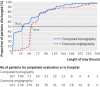
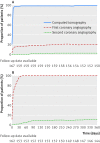
Results: Cardiac CT reduced the need for coronary angiography from 100% to 14% (95% confidence interval 9% to 20%, P<0.001) and was associated with a significantly greater diagnostic yield from coronary angiography: 75% (53% to 90%) v 15% (10% to 22%), P<0.001. Major procedural complications were uncommon (0.3%) and similar across groups. Minor procedural complications were less common in the CT group than in the coronary angiography group: 3.6% (1% to 8%) v 10.5% (6% to 16%), P=0.014. CT shortened the median length of stay in the angiography group from 52.9 hours (interquartile range 49.5-76.4 hours) to 30.0 hours (3.5-77.3 hours, P<0.001). Overall median exposure to radiation was similar between the CT and angiography groups: 5.0 mSv (interquartile range 4.2-8.7 mSv) v 6.4 mSv (3.4-10.7 mSv), P=0.45. After a median follow-up of 3.3 years, major adverse cardiovascular events had occurred in seven of 167 patients in the CT group (4.2%) and six of 162 (3.7%) in the coronary angiography group (adjusted hazard ratio 0.90, 95% confidence interval 0.30 to 2.69, P=0.86). 79% of patients stated that they would prefer CT for subsequent testing. The study was conducted at a University hospital in Germany and thus the performance of CT may be different in routine clinical practice. The prevalence was lower than expected, resulting in an underpowered study for the predefined primary outcome.
Conclusions: CT increased the diagnostic yield and was a safe gatekeeper for coronary angiography with no increase in long term events. The length of stay was shortened by 22.9 hours with CT, and patients preferred non-invasive testing.Trial registration ClinicalTrials.gov NCT00844220.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi-disclosure.pdf (available on request from the corresponding author) and declare that (1) MD has support from the Heisenberg programme of the German Research Foundation for the submitted work; (2) MD has relationships with Bayer, Bracco, Cardiac MR Academy, European Commission, European Regional Development Fund, German Foundation of Heart Research, German Federal Ministry of Education and Research, GE Healthcare, Guerbet, Springer, and Toshiba; BH has relationships with Bayer, Bracco, GE, Guerbet, Philips, Siemens, and Toshiba; (3) their spouses, partners, or children have no financial relationships that may be relevant to the submitted work; and (4) have no non-financial interests that may be relevant to the submitted work. Institutional master research agreements exist with Siemens Medical Solutions, Philips Medical Systems, and Toshiba Medical Systems. The terms of these arrangements are managed by the legal department of Charité–Universitätsmedizin Berlin.
Figures


References
-
- Montalescot G, Sechtem U, Achenbach S, et al. Task Force Members ESC Committee for Practice Guidelines Document Reviewers. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949-3003. 10.1093/eurheartj/eht296. pmid:23996286. - DOI - PubMed
-
- Noto TJ Jr, , Johnson LW, Krone R, et al. Cardiac catheterization 1990: a report of the Registry of the Society for Cardiac Angiography and Interventions (SCA&I). Cathet Cardiovasc Diagn 1991;24:75-83. 10.1002/ccd.1810240202 pmid:1742788. - DOI - PubMed
-
- Levenson B, Albrecht A, Göhring S, et al. QuIK-Register des Bundesverbandes Niedergelassener Kardiologen (BNK). [6th report of the German Association of Cardiologists in private practice (BNK) on quality assurance in cardiac catheterization and coronary intervention 2006–2009]. Herz 2011;36:41-9. 10.1007/s00059-011-3423-x. pmid:21308430. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
