Identification of peptides, metal binding and lipid peroxidation activities of HPLC fractions of hydrolyzed oat bran proteins
- PMID: 27777466
- PMCID: PMC5069264
- DOI: 10.1007/s13197-016-2341-6
Identification of peptides, metal binding and lipid peroxidation activities of HPLC fractions of hydrolyzed oat bran proteins
Abstract
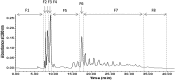
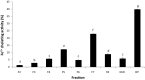
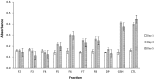
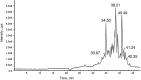
The aim of this study was to evaluate metal binding and antioxidant activities of hydrolyzed oat bran proteins followed by the determination of peptide sequences. Protamex oat bran protein hydrolysates (OBPH) were separated by reverse-phase HPLC into eight peptide fractions (F1-F8) and their abilities to either chelate metals (Fe2+, Ca2+) or prevent the oxidation of lipids were investigated. In the Fe2+ chelation assay, OBPH had significantly (p < 0.05) higher activity (39.7 %) than the best performed fraction F7 (22.8 %). The second most active was F5 with 12.1 % chelating activity and this was higher than the activity of the tripeptide glutathione (5.8 %) used as control. The two most Fe2+ chelating fractions (F5, F7) however had weak calcium binding (0.6-1.0 %) properties at peptide concentration ranging from 0.2 to 1.0 mg/mL. In the lipid peroxidation assay, OBPH and all HPLC fractions prevented the oxidation of linoleic acid. More than 60 peptides mainly derived from globulin and avenin proteins were identified using tandem mass spectrometry.
Keywords: Calcium binding; Mass spectrometry; Metal chelation; Oat bran; Protein hydrolysate.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
Similar articles
-
Peptidomic analysis of hydrolyzed oat bran proteins, and their in vitro antioxidant and metal chelating properties.Food Chem. 2019 May 1;279:49-57. doi: 10.1016/j.foodchem.2018.11.110. Epub 2018 Dec 6. Food Chem. 2019. PMID: 30611511
-
Isolation and identification of antioxidant peptides from enzymatically hydrolyzed rice bran protein.Food Chem. 2016 Feb 1;192:156-62. doi: 10.1016/j.foodchem.2015.06.057. Epub 2015 Jun 19. Food Chem. 2016. PMID: 26304333
-
Isolation and Identification of Tyrosinase-Inhibitory and Copper-Chelating Peptides from Hydrolyzed Rice-Bran-Derived Albumin.J Agric Food Chem. 2018 Aug 8;66(31):8346-8354. doi: 10.1021/acs.jafc.8b01849. Epub 2018 Jul 31. J Agric Food Chem. 2018. PMID: 30016586 Free PMC article.
-
Amended final report on the safety assessment of Oryza Sativa (rice) Bran Oil, Oryza Sativa (rice) Germ Oil, Rice Bran Acid,Oryza Sativa (rice) Bran Wax, Hydrogenated Rice Bran Wax, Oryza Sativa (rice)Bran Extract, Oryza Sativa (rice) Extract, Oryza Sativa (rice) Germ Powder, Oryza Sativa (rice) Starch, Oryza Sativa (rice) Bran, Hydrolyzed Rice Bran Extract, Hydrolyzed Rice Bran Protein, Hydrolyzed Rice Extract, and Hydrolyzed Rice Protein.Int J Toxicol. 2006;25 Suppl 2:91-120. doi: 10.1080/10915810600964626. Int J Toxicol. 2006. PMID: 17090480 Review.
-
Antioxidant properties and potential mechanisms of hydrolyzed proteins and peptides from cereals.Heliyon. 2019 Apr 29;5(4):e01538. doi: 10.1016/j.heliyon.2019.e01538. eCollection 2019 Apr. Heliyon. 2019. PMID: 31183417 Free PMC article. Review.
Cited by
-
Production of antioxidant peptide fractions from a by-product of tomato processing: mass spectrometry identification of peptides and stability to gastrointestinal digestion.J Food Sci Technol. 2018 Sep;55(9):3498-3507. doi: 10.1007/s13197-018-3274-z. Epub 2018 Jul 7. J Food Sci Technol. 2018. PMID: 30150808 Free PMC article.
-
Impact of Nitrite Supplementation on Bioactive Peptides during Sausage Processing.Foods. 2023 Jan 14;12(2):407. doi: 10.3390/foods12020407. Foods. 2023. PMID: 36673498 Free PMC article.
-
Peptidomics Analysis of Soy Protein Hydrolysates-Antioxidant Properties and Mechanism of their Inhibition of the Oxidation of Palm Olein during Frying Cycles.Foods. 2023 Sep 20;12(18):3498. doi: 10.3390/foods12183498. Foods. 2023. PMID: 37761207 Free PMC article.
-
Antioxidant and Anti-Apoptotic Properties of Oat Bran Protein Hydrolysates in Stressed Hepatic Cells.Foods. 2019 May 11;8(5):160. doi: 10.3390/foods8050160. Foods. 2019. PMID: 31083557 Free PMC article.
-
In Vitro Studies of Fermented Korean Chung-Yang Hot Pepper Phenolics as Inhibitors of Key Enzymes Relevant to Hypertension and Diabetes.Foods. 2019 Oct 14;8(10):498. doi: 10.3390/foods8100498. Foods. 2019. PMID: 31615144 Free PMC article.
References
-
- Bao XL, Song M, Zhang J, et al. Calcium-binding ability of soy protein hydrolysates. Chin Chem Lett. 2007;18:1115–1118. doi: 10.1016/j.cclet.2007.07.032. - DOI
-
- Brieger K, Schiavone S, Miller FJ, Krause K-H. Reactive oxygen species: from health to disease. Swiss Med Wkly. 2012;142:w13659. - PubMed
-
- Cariolou MA, Morse DE. Purification and characterization of calcium-binding conchiolin shell peptides from the mollusc, Haliotis rufescens, as a function of development. J Comp Physiol B. 1988;157:717–729. doi: 10.1007/BF00691002. - DOI
-
- Chang Y-W, Alli I, Konishi Y, Ziomek E. Characterization of protein fractions from chickpea (Cicer arietinum L.) and oat (Avena sativa L.) seeds using proteomic techniques. Food Res Int. 2011;44:3094–3104.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
