Efficient hydrolysis of raw starch and ethanol fermentation: a novel raw starch-digesting glucoamylase from Penicillium oxalicum
- PMID: 27777618
- PMCID: PMC5069817
- DOI: 10.1186/s13068-016-0636-5
Efficient hydrolysis of raw starch and ethanol fermentation: a novel raw starch-digesting glucoamylase from Penicillium oxalicum
Abstract
Background: Starch is a very abundant and renewable carbohydrate and is an important feedstock for industrial applications. The conventional starch liquefaction and saccharification processes are energy-intensive, complicated, and not environmentally friendly. Raw starch-digesting glucoamylases are capable of directly hydrolyzing raw starch to glucose at low temperatures, which significantly simplifies processing and reduces the cost of producing starch-based products.
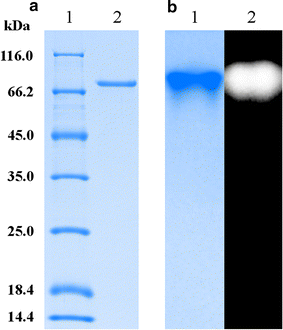
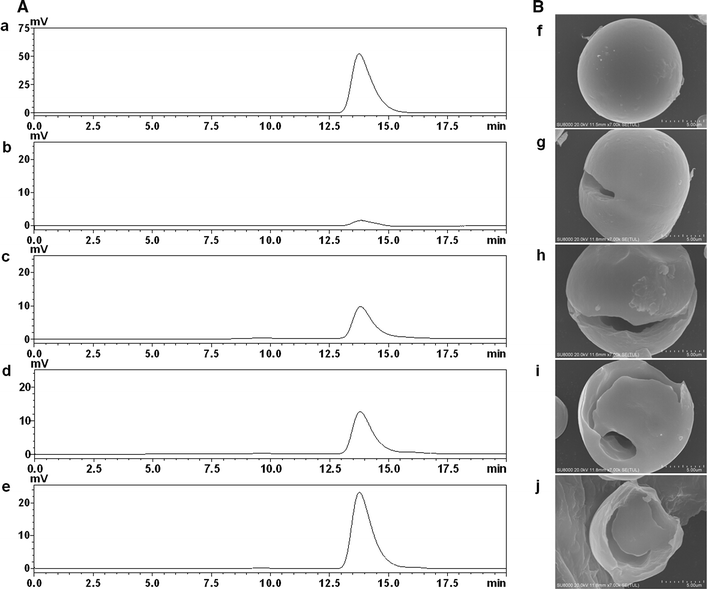
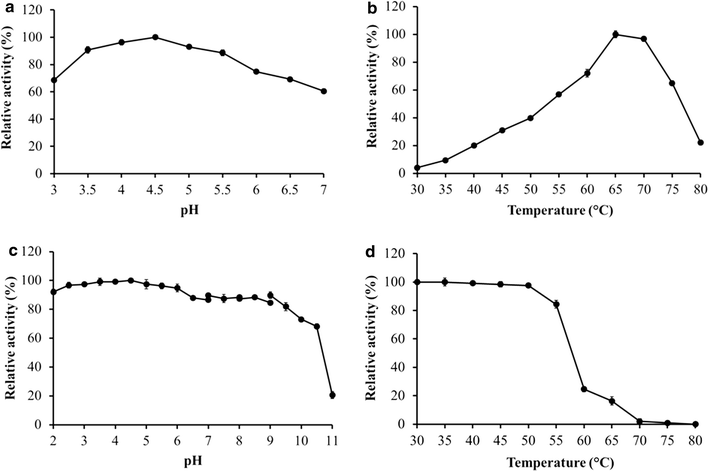
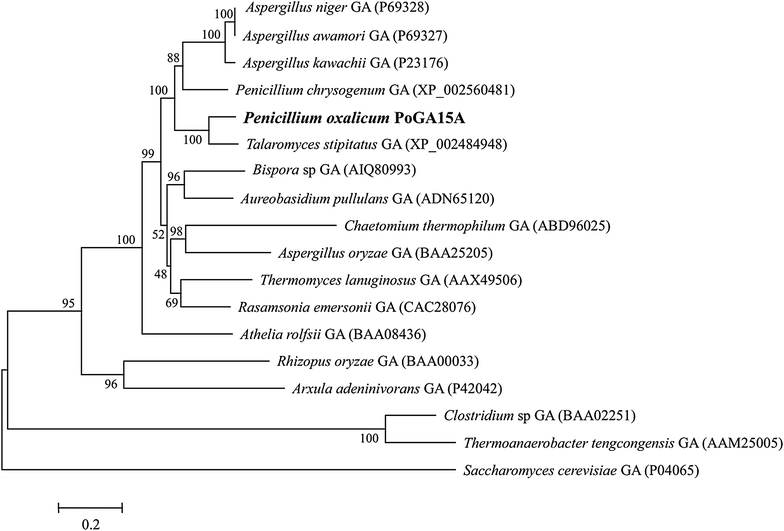
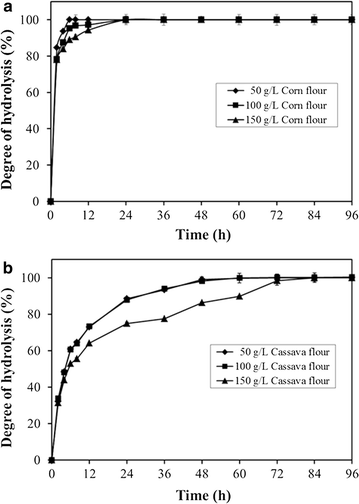
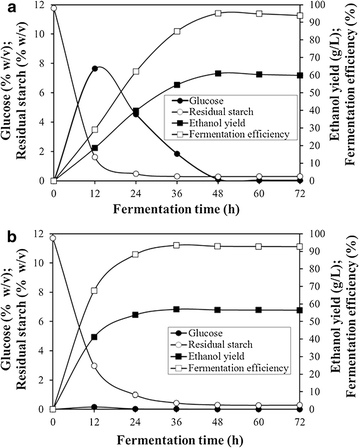
Results: A novel raw starch-digesting glucoamylase PoGA15A with high enzymatic activity was purified from Penicillium oxalicum GXU20 and biochemically characterized. The PoGA15A enzyme had a molecular weight of 75.4 kDa, and was most active at pH 4.5 and 65 °C. The enzyme showed remarkably broad pH stability (pH 2.0-10.5) and substrate specificity, and was able to degrade various types of raw starches at 40 °C. Its adsorption ability for different raw starches was consistent with its degrading capacities for the corresponding substrate. The cDNA encoding the enzyme was cloned and heterologously expressed in Pichia pastoris. The recombinant enzyme could quickly and efficiently hydrolyze different concentrations of raw corn and cassava flours (50, 100, and 150 g/L) with the addition of α-amylase at 40 °C. Furthermore, when used in the simultaneous saccharification and fermentation of 150 g/L raw flours to ethanol with the addition of α-amylase, the ethanol yield reached 61.0 g/L with a high fermentation efficiency of 95.1 % after 48 h when raw corn flour was used as the substrate. An ethanol yield of 57.0 g/L and 93.5 % of fermentation efficiency were achieved with raw cassava flour after 36 h. In addition, the starch-binding domain deletion analysis revealed that SBD plays a very important role in raw starch hydrolysis by the enzyme PoGA15A.
Conclusions: A novel raw starch-digesting glucoamylase from P. oxalicum, with high enzymatic activity, was biochemically, molecularly, and genetically identified. Its efficient hydrolysis of raw starches and its high efficiency during the direct conversion of raw corn and cassava flours via simultaneous saccharification and fermentation to ethanol suggests that the enzyme has a number of potential applications in industrial starch processing and starch-based ethanol production.
Keywords: Cassava starch; Corn starch; Ethanol; Gene cloning and expression; Penicillium oxalicum; Raw starch hydrolysis; Raw starch-digesting glucoamylase; Simultaneous saccharification and fermentation.
Figures
Similar articles
-
Production of raw cassava starch-degrading enzyme by Penicillium and its use in conversion of raw cassava flour to ethanol.J Ind Microbiol Biotechnol. 2011 Jun;38(6):733-42. doi: 10.1007/s10295-010-0910-7. Epub 2010 Dec 1. J Ind Microbiol Biotechnol. 2011. PMID: 21120680
-
Enhanced production of raw starch degrading enzyme using agro-industrial waste mixtures by thermotolerant Rhizopus microsporus for raw cassava chip saccharification in ethanol production.Prep Biochem Biotechnol. 2017 Sep 14;47(8):813-823. doi: 10.1080/10826068.2017.1342264. Epub 2017 Jun 21. Prep Biochem Biotechnol. 2017. PMID: 28636431
-
Direct fermentation of raw starch using a Kluyveromyces marxianus strain that expresses glucoamylase and alpha-amylase to produce ethanol.Biotechnol Prog. 2014 Mar-Apr;30(2):338-47. doi: 10.1002/btpr.1877. Epub 2014 Feb 12. Biotechnol Prog. 2014. PMID: 24478139
-
Engineering Saccharomyces cerevisiae for direct conversion of raw, uncooked or granular starch to ethanol.Crit Rev Biotechnol. 2015;35(3):369-91. doi: 10.3109/07388551.2014.888048. Crit Rev Biotechnol. 2015. PMID: 24666118 Review.
-
Biocatalysis for the production of industrial products and functional foods from rice and other agricultural produce.J Agric Food Chem. 2008 Nov 26;56(22):10445-51. doi: 10.1021/jf801928e. J Agric Food Chem. 2008. PMID: 18942836 Review.
Cited by
-
Enhanced saccharification levels of corn starch using as a strategy a novel amylolytic complex (AmyHb) from the thermophilic fungus Humicola brevis var. thermoidea in association with commercial enzyme.3 Biotech. 2024 Sep;14(9):198. doi: 10.1007/s13205-024-04038-y. Epub 2024 Aug 8. 3 Biotech. 2024. PMID: 39131173
-
Artificially selecting bacterial communities using propagule strategies.Evolution. 2020 Oct;74(10):2392-2403. doi: 10.1111/evo.14092. Epub 2020 Sep 15. Evolution. 2020. PMID: 32888315 Free PMC article.
-
AmyZ1: a novel α-amylase from marine bacterium Pontibacillus sp. ZY with high activity toward raw starches.Biotechnol Biofuels. 2019 Apr 23;12:95. doi: 10.1186/s13068-019-1432-9. eCollection 2019. Biotechnol Biofuels. 2019. PMID: 31044008 Free PMC article.
-
Single-step ethanol production from raw cassava starch using a combination of raw starch hydrolysis and fermentation, scale-up from 5-L laboratory and 200-L pilot plant to 3000-L industrial fermenters.Biotechnol Biofuels. 2021 Mar 16;14(1):68. doi: 10.1186/s13068-021-01903-3. Biotechnol Biofuels. 2021. PMID: 33726825 Free PMC article.
-
Simultaneous saccharification and ethanologenic fermentation (SSF) of waste bread by an amylolytic Parageobacillus thermoglucosidasius strain TM333.Microb Cell Fact. 2022 Nov 28;21(1):251. doi: 10.1186/s12934-022-01971-6. Microb Cell Fact. 2022. PMID: 36443865 Free PMC article.
References
-
- Vamadevan V, Bertoft E. Structure-function relationships of starch components. Starch/Stärke. 2015;67:55–68. doi: 10.1002/star.201400188. - DOI
-
- Waterschoot J, Gomand SV, Fierens E, Delcour JA. Production, structure, physicochemical and functional properties of maize, cassava, wheat, potato and rice starches. Starch/Stärke. 2015;67:14–29. doi: 10.1002/star.201300238. - DOI
-
- Favaro L, Viktor MJ, Rose SH, Viljoen-Bloom M, van Zyl WH, Basaglia M, Cagnin L, Casella S. Consolidated bioprocessing of starchy substrates into ethanol by industrial Saccharomyces cerevisiae strains secreting fungal amylases. Biotechnol Bioeng. 2015;112:1751–1760. doi: 10.1002/bit.25591. - DOI - PubMed
-
- Sharma A, Satyanarayana T. Microbial acid-stable α-amylases: characteristics, genetic engineering and applications. Process Biochem. 2013;48:201–211. doi: 10.1016/j.procbio.2012.12.018. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

